Abstract
Background
The treatment of hyperlipidaemia in human immunodeficiency virus (HIV)-infected patients has become increasingly important. However, treatment options are limited because of the drug–drug interaction between certain statins and HIV medications metabolized by cytochrome P450 (CYP) enzymes.
Objectives
The primary objective was to investigate the steady-state pharmacokinetics of pitavastatin when co-administered with darunavir/ritonavir. The secondary objective was to investigate the steady-state pharmacokinetics of both darunavir and ritonavir when co-administered with pitavastatin.
Methods
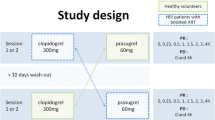
This was a single-centre, open-label, multi-dose, fixed-sequence study in HIV seronegative healthy volunteers. Pitavastatin 4 mg was administered once daily on days 1–5 and on days 12–16, and darunavir 800 mg/ritonavir 100 mg once daily on days 6–16. Pharmacokinetic blood sampling was performed on days 5, 11 and 16. No significant interaction was concluded if the 90 % confidence intervals (CIs) of the geometric mean ratios (GMRs) for total exposure [i.e. the area under the plasma concentration–time curve over a dosing interval at steady state (AUC0–τ )] and for peak exposure [i.e. the maximum plasma concentration (C max)] of the two treatments were within the 80–125 % range.
Results
Twenty-eight subjects (mean age 30.5 years) were enrolled, and pharmacokinetic data were available for 27 subjects. For pitavastatin, the GMRs and 90 % CIs for the AUC0–τ and C max ratios with co-administration were 0.74 (0.69–0.80) and 0.96 (0.84–1.09), respectively. For both darunavir and ritonavir, the 90 % CIs for the AUC0–τ and C max ratios were within 80–125 % with pitavastatin co-administration. No significant safety issues were reported.
Conclusion
Darunavir/ritonavir decreased total exposure to pitavastatin by 26 %, while peak exposures were similar. Pitavastatin did not influence the pharmacokinetics of darunavir or ritonavir. There is limited interaction between pitavastatin and darunavir/ritonavir.


Similar content being viewed by others
References
Global AIDS Overview. http://aids.gov/federal-resources/around-the-world/global-aids-overview/. Accessed 17 Apr 2014.
World Health Organization, Europe, Data and statistics. http://www.euro.who.int/en/health-topics/communicable-diseases/hivaids/data-and-statistics. Accessed 17 Apr 2014.
HIV in the United States: at a glance. http://www.cdc.gov/hiv/resources/factsheets/us.htm. Accessed 17 Apr 2014.
Crum NF, Riffenburgh RH, Wegner S, et al for the Triservice AIDS Clinical Consortium. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006;41:194–200.
Palella FJ Jr, Baker RK, Moorman AC, et al., for the HIV Outpatient Study Investigators. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27-34.
Go AS, Mozaffarian D, Roger VL, et al on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292.
Félix-Redondo FJ, Grau M, Fernández-Bergés D. Cholesterol and cardiovascular disease in the elderly. Facts and gaps. Aging Dis. 2013;4:154–69.
Giannarelli C, Klein RS, Badimon JJ. Cardiovascular implications of HIV-induced dyslipidemia. Atherosclerosis. 2011;219:384–9.
Dubé MP, Cadden JJ. Lipid metabolism in treated HIV infection. Best Pract Res Clin Endocrinol Metab. 2011;25:429–42.
Chauvin B, Drouot S, Barrail-Tran A, Taburet AM. Drug–drug interactions between HMG-CoA reductase inhibitors (statins) and antiviral protease inhibitors. Clin Pharmacokinet. 2013;52:815–31.
FDA Drug Safety Communication. Interactions between certain HIV or hepatitis C drugs and cholesterol-lowering statin drugs can increase the risk of muscle injury, March 1, 2012. Available from http://www.fda.gov/Drugs/DrugSafety/ucm293877.htm. Accessed 17 Oct 2013.
Duggan ST. Pitavastatin: a review of its use in the management of hypercholesterolemia or mixed dyslipidemia. Drugs. 2012;72:565–84.
Hirano M, Maeda K, Shitara Y, et al. Contribution of OATP2 (OATP1B1) and OATP8 (OATP1B3) to the hepatic uptake of pitavastatin in humans. J Pharmacol Exp Ther. 2004;311:139–46.
Hirano M, Maeda K, Shitara Y, et al. Drug–drug interaction between pitavastatin and various drugs via OATP1B1. Drug Metab Dispos. 2006;34:1229–36.
Choi MK, Shin HJ, Choi YL, et al. Differential effect of genetic variants of Na(+)-taurocholate co-transporting polypeptide (NTCP) and organic anion-transporting polypeptide 1B1 (OATP1B1) on the uptake of HMG-CoA reductase inhibitors. Xenobiotica. 2011;41:24–34.
Prezista® (darunavir): package insert. Janssen Pharmaceuticals, Inc., Titusville, NJ; 2013.
Morgan RE, Campbell SE, Suehira K, et al. Effects of steady-state lopinavir/ritonavir on the pharmacokinetics of pitavastatin in healthy adult volunteers. J Acquir Immune Defic Syndr. 2012;60:158–64.
Abd TT, Jacobson TA. Statin-induced myopathy: a review and update. Expert Opin Drug Saf. 2011;10:373–87.
Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol. 2005;19:117–25.
Fujino H, Yamada I, Shimada S, et al. Metabolic fate of pitavastatin, a new HMG-CoA reductase: human UDP-glucuronosyltransferase enzymes involved in lactonization. Xenobiotica. 2003;33:27–41.
Foisy MM, Yakiwchuk EM, Hughes CA. Induction effects of ritonavir: implications for drug interactions. Ann Pharmacother. 2008;42:1048–59.
Samineni D, Desai PB, Sallans L, Fichtenbaum CJ. Steady-state pharmacokinetic interactions of darunavir/ritonavir with lipid-lowering agent rosuvastatin. J Clin Pharmacol. 2012;52:922–31.
Sekar VJ, Spinosa-Guzman S, Marien K, et al. Pharmacokinetic drug–drug interaction between the new HIV protease inhibitor darunavir (TMC114) and the lipid-lowering agent pravastatin. Eighth International Workshop on Clinical Pharmacology of HIV Therapy. 16–18 April 2007, Budapest, Hungary. Abstract 54
Aquilante CL, Kiser JJ, Anderson PL, et al. Influence of SLCO1B1 polymorphisms on the drug–drug interaction between darunavir/ritonavir and pravastatin. J Clin Pharmacol. 2012;52:1725–38.
Nakaya N, Soji N, Kaneko T, et al. Efficacy and safety of NK-104 (pitavastatin), a novel HMG-CoA reductase inhibitor in hypercholesterolemic volunteers. J Clin Ther Med. 2001;17:767–87.
Mitsuya Y, Liu TF, Rhee SY, et al. Prevalence of darunavir resistance-associated mutations: patterns of occurrence and association with past treatment. J Infect Dis. 2007;196:1177–9.
De Meyer SM, Spinosa-Guzman S, Vangeneugden TJ, et al. Efficacy of once-daily darunavir/ritonavir 800/100 mg in HIV-infected, treatment-experienced patients with no baseline resistance-associated mutations to darunavir. J Acquir Immune Defic Syndr. 2008;49:179–82.
Acknowledgments
The authors wish to acknowledge Lorraine R. Baer, PharmD, of Baer PharMed Consulting, for assistance with the writing and editing of the manuscript.
Sources of Funding
Kowa Pharmaceuticals America, Inc. (Montgomery, AL, USA) and Eli Lilly and Company (Indianapolis, IN, USA).
Disclosure Statement
C. Yu and D. Small are employees of, and have stock ownership in, Eli Lilly and Company; S. Campbell and R. Morgan are employees of Kowa Research Institute, Inc.; C. Sponseller is an employee of Kowa Pharmaceuticals America, Inc.; M. Medlock is an independent contractor employed by PPD, Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Clinical trials.gov registration number: NCT01422369.
Rights and permissions
About this article
Cite this article
Yu, C.Y., Campbell, S.E., Sponseller, C.A. et al. Steady-State Pharmacokinetics of Darunavir/Ritonavir and Pitavastatin when Co-administered to Healthy Adult Volunteers. Clin Drug Investig 34, 475–482 (2014). https://doi.org/10.1007/s40261-014-0198-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-014-0198-x