Abstract
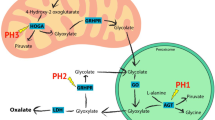
Over the last few years, US Food and Drug Administration-approved drugs using RNA interference have come to the market. Many have treated liver-specific diseases utilizing N-acetyl galactosamine conjugation because of its effective delivery and limited off-target effects. The autosomal recessive disorder primary hyperoxaluria, specifically type 1, has benefited from these developments. Primary hyperoxaluria arises from mutations in the enzymes involved in endogenous oxalate synthesis. The severity of disease varies but can result in kidney failure and systemic oxalosis. Until recently, the treatment options were limited and focused primarily on supportive treatments, pyridoxine use in a subset of patients with primary hyperoxaluria type 1, and liver-kidney transplants in those who progressed to kidney failure. Two genes have been targeted with RNA interference; lumasiran targets glycolate oxidase and nedosiran targets lactate dehydrogenase A. Lumasiran was recently approved in the treatment of primary hyperoxaluria type 1 and nedosiran is in the approval process. Unfortunately, despite initial hopes that nedosiran may also be a treatment option for primary hyperoxaluria types 2 and 3, initial data suggest otherwise. The use of RNA interference liver-specific targeting for the treatment of primary hyperoxaluria type 1 will likely transform the natural history of the disease.

Similar content being viewed by others
References
Holmes RP, Assimos DG. Glyoxylate synthesis, and its modulation and influence on oxalate synthesis. J Urol. 1998;160(5):1617–24.
Burns Z, Knight J, Fargue S, et al. Future treatments for hyperoxaluria. Curr Opin Urol. 2020;30(2):171–6.
Liebow A, Li X, Racie T, et al. An investigational RNAi therapeutic targeting glycolate oxidase reduces oxalate production in models of primary hyperoxaluria. J Am Soc Nephrol. 2017;28:494–503.
Lai C, Pursell N, Gierut J, et al. Specific inhibition of hepatic lactate dehydrogenase reduces oxalate production in mouse models of primary hyperoxaluria. Mol Ther. 2018;26(8):1983–95.
Wood KD, Holmes RP, Erbe D, et al. Reduction in urinary oxalate excretion in mouse models of primary hyperoxaluria by RNA interference inhibition of liver lactate dehydrogenase activity. Biochim Biophys Acta Mol Basis Dis. 2019;1865(9):2203–9.
Frishberg Y, Zeharia A, Lyakhovetsky R, Bargal R, Belostotsky R. Mutations in HAO1 encoding glycolate oxidase cause isolated glycolic aciduria. J Med Genet. 2014;51(8):526–9. https://doi.org/10.1136/jmedgenet-2014-102529.
McGregor TL, Hunt KA, Yee E, et al. Characterising a healthy adult with a rare HAO1 knockout to support a therapeutic strategy for primary hyperoxaluria. Elife. 2020;9:e54363.
Kanno T, Sudo K, Takeuchi I, et al. Hereditary deficiency of lactate dehydrogenase M-subunit. Clin Chim Acta. 1980;108(2):267–76.
Nishimura Y, Honda N, Ohyama K, et al. Lactate dehydrogenase A subunit deficiency. Isozymes Curr Top Biol Med Res. 1983;11:51–64.
Maekawa M, Kanda S, Sudo K, et al. Estimation of the gene frequency of lactate dehydrogenase subunit deficiencies. Am J Hum Genet. 1984;36(6):1204–14.
Kanno T, Sudo K, Maekawa M, et al. Lactate dehydrogenase M-subunit deficiency: a new type of hereditary exertional myopathy. Clin Chim Acta. 1988;173(1):89–98.
Maekawa M, Kanno T, Sudo K. Myoglobinuria due to enzyme abnormalities in glycolytic pathway: especially lactate dehydrogenase M subunit deficiency. Rinsho Byori. 1991;39(2):124–32.
Ariceta G, Barrios K, Brown BD, Hoppe B, Rosskamp R, Langman CB. Hepatic lactate dehydrogenase A: an RNA interference target for the treatment of all known types of primary hyperoxaluria. Kidney Int Rep. 2021;6:1088–98.
Garrelfs SF, Frishberg Y, Hulton SA, et al. Lumasiran, an RNAi therapeutic for primary hyperoxaluria type 1. N Engl J Med. 2021;384:1216–26.
Cochat P, Rumsby G. Primary hyperoxaluria. N Engl J Med. 2013;369(7):649–58.
Salido E, Pey AL, Rodriguez R, et al. Primary hyperoxalurias: disorders of glyoxylate detoxification. Biochim Biophys Acta. 2012;1822(9):1453–64.
Lieske JC, Monico CG, Holmes WS, et al. International registry for primary hyperoxaluria. Am J Nephrol. 2005;25(3):290–6. https://doi.org/10.1159/000086360.
Hoppe B. An update on primary hyperoxaluria. Nat Rev Nephrol. 2012;8(8):467–75. https://doi.org/10.1038/nrneph.2012.113.
Cochat P, Hulton SA, Acquaviva C, et al. Primary hyperoxaluria type 1: indications for screening and guidance for diagnosis and treatment. Nephrol Dial Transplant. 2012;27(5):1729–36. https://doi.org/10.1093/ndt/gfs078.
Bergstralh EJ, Monico CG, Lieske JC, et al. Transplantation outcomes in primary hyperoxaluria. Am J Transplant. 2010;10(11):2493–501. https://doi.org/10.1111/j.1600-6143.2010.03271.x.
Cramer SD, Ferree PM, Lin K, et al. The gene encoding hydroxypyruvate reductase (GRHPR) is mutated in patients with primary hyperoxaluria type II. Hum Mol Genet. 1999;11:2063–9.
Monico CG, Rossetti S, Belostotsky R, et al. Primary hyperoxaluria type III gene HOGA1 (formerly DHDPSL) as a possible risk factor for idiopathic calcium oxalate urolithiasis. Clin J Am Soc Nephrol. 2011;6(9):2289–95.
Belostotsky R, Seboun E, Idelson GH, et al. Mutations in DHDPSL are responsible for primary hyperoxaluria type III. Am J Hum Genet. 2010;87:392–9.
Fire A, Xu S, Montgomery MK, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–11.
Hannon GJ. RNA interference. Nature. 2002;418(6894):244–51.
Nair JK, Willoughby JL, Chan A, et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc. 2014;136(49):16958–61.
Wu YT, Jiaang WT, Lin KG, et al. A new N-acetylgalactosamine containing peptide as a targeting vehicle for mammalian hepatocytes via asialoglycoprotein receptor endocytosis. Curr Drug Deliv. 2004;1(2):119–27.
Bobbin ML, Rossi JJ. RNA interference (RNAi)-based therapeutics: delivering on the promise? Annu Rev Pharmacol Toxicol. 2016;56:103–22. https://doi.org/10.1146/annurev-pharmtox-010715-103633.
Scott LJ, Keam SJ. Lumasiran: first approval. Drugs. 2021;81:277–82.
Frishberg Y, Deschênes G, Groothoff JW, et al. Phase 1/2 study of lumasiran for treatment of primary hyperoxaluria type 1: a placebo-controlled randomized clinical trial. Clin J Am Soc Nephrol. 2021;16:1025–36.
Sas DJ, Magen D, Hayes W, et al. Phase 3 trial of lumasiran for primary hyperoxaluria type 1: a new RNAi therapeutic in infants and young children. Genet Med. 2022;24(3):654–62.
Dicerna Pharmaceuticals, Inc. Dicerna reports positive top-line results from PHYOX™2 pivotal clinical trial of nedosiran for the treatment of primary hyperoxaluria. 2021. https://investors.dicerna.com/news-releases/news-release-details/dicerna-reports-positive-top-line-results-phyoxtm2-pivotal. Accessed 15 Mar 2022.
Hoppe B, Koch A, Cochat P, et al. Safety, pharmacodynamics, and exposure-response modeling results from a first-in-human phase 1 study of nedosiran (PHOX1) in primary hyperoxaluria. Kidney Int. 2022;101(3):626–34.
Dicerna Pharmaceuticals, Inc. Dicerna announces results for PHYOX™4, single-dose study of nedosiran in primary hyperoxaluria type 3 (PH3). 2021. https://www.businesswire.com/news/home/20211019005355/en/. Accessed 15 Mar 2022.
Hulton SA, Groothoff JW, Frishberg Y, et al. Randomized clinical Trial on the long-term efficacy and safety of lumasiran in patients with primary hyperoxaluria type 1. Kidney Int Rep. 2021;7(3):494–506. https://doi.org/10.1016/j.ekir.2021.12.001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest/Competing Interests
Kyle Wood reports consulting fees and is on the scientific advisory board for Alnylam Pharmaceuticals. All other authors report no potential conflicts of interest or financial disclosures that are pertinent to the article.
Ethics Approval
Ethics approval is not applicable to this article as no human or animal subjects were analyzed in this review.
Consent to Participate
Consent to participate is not applicable to this article as no human or animal subjects were analyzed in this review.
Consent for Publication
Consent for publication is not applicable to this article as no human or animal subjects were analyzed in this review.
Availability of Data and Material
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Code Availability
This is not applicable to this article as no new data were created or analyzed with code in this study.
Authors’ Contributions
All authors contributed to the literature review, writing, and editing of the article.
Rights and permissions
About this article
Cite this article
Sawyer, K., Leahy, S. & Wood, K.D. Progress with RNA Interference for the Treatment of Primary Hyperoxaluria. BioDrugs 36, 437–441 (2022). https://doi.org/10.1007/s40259-022-00539-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-022-00539-5