Abstract
Aim
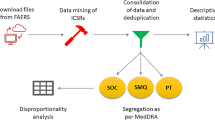
The aim of this article was to provide an overview of adverse events reported for erenumab in post-marketing through the Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) and perform a disproportionality analysis with other drugs used for acute or preventative treatment of migraine as controls.
Methods
FAERS was screened from the first quarter of 2018 to the second quarter of 2020 (latest data update 30 June 2020). Clinical and demographic characteristics of cases were described along with the seriousness and outcome of adverse events. Disproportionality analyses were performed using the reporting odds ratio (ROR).
Results
In total, 23,312 cases were reported during the study period, 67.0% by consumers. Cases in the age range 18–64 years (10,922 cases; 45.8%), in female sex (15,099 cases; 64.8%), and with adverse events that were classified as non-serious (19,626 cases; 84.2%) were the most prevalent in the database. After the exclusion of duplicates, 146 fatal cases were identified. A total of 1303 unlabeled adverse events were reported, of which 49 had statistically significant disproportionality of reporting in comparison with other drugs used for acute or preventative treatment of migraine. Identified disproportionality signals included alopecia, depression, anxiety, myocardial infarction, increased heart rate, pulmonary embolism, weight alteration, insomnia, tinnitus, and influenza-like symptoms. Injection-site reactions (labeled events) were co-reported with errors in administration procedures.
Conclusion
Adverse events reported during the first 2 years of post-marketing surveillance were mostly non-serious and with a favorable prognosis. However, new safety aspects emerged for which further studies are needed to confirm the associations, prioritizing unlabeled events with consistent disproportionality signals (e.g., emerging in at least 4 out of 6 analyses).





Similar content being viewed by others
References
King CT, Gegg CV, Hu SN, Sen Lu H, Chan BM, Berry KA, Brankow DW, Boone TJ, Kezunovic N, Kelley MR, Shi L, Xu C. Discovery of the Migraine Prevention Therapeutic Aimovig (Erenumab), the First FDA-Approved Antibody against a G-Protein-Coupled Receptor. ACS Pharmacol Transl Sci. 2019;2(6):485–90. https://doi.org/10.1021/acsptsci.9b00061.
European Medicine Agency. Aimovig [Internet]. https://www.ema.europa.eu/en/documents/product-information/aimovig-epar-product-information_en.pdf. Accessed 11 Oct 2020.
Garland SG, Smith SM, Gums JG. Erenumab: a first-in-class monoclonal antibody for migraine prevention. Ann Pharmacother. 2019;53:933–9.
Chaplin S. Erenumab: a monoclonal antibody for migraine prophylaxis. Prescriber. 2019;30:38–9.
Dodick DW, Ashina M, Brandes JL, Kudrow D, Lanteri-Minet M, Osipova V, et al. ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38:1026–37.
Goadsby PJ, Reuter U, Hallström Y, Broessner G, Bonner JH, Zhang F, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123–32.
Reuter U, Goadsby PJ, Lanteri-Minet M, Wen S, Hours-Zesiger P, Ferrari MD, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet. 2018;392:2280–7.
Sun H, Dodick DW, Silberstein S, Goadsby PJ, Reuter U, Ashina M, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15:382–90.
Tepper S, Ashina M, Reuter U, Brandes JL, Doležil D, Silberstein S, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16:425–34.
Sakai F, Takeshima T, Tatsuoka Y, Hirata K, Lenz R, Wang Y, et al. A randomized phase 2 study of erenumab for the prevention of episodic migraine in Japanese adults. Headache J Head Face Pain. 2019;59:1731–42.
Goadsby PJ, Reuter U, Hallström Y, Broessner G, Bonner JH, Zhang F, et al. One-year sustained efficacy of erenumab in episodic migraine: results of the STRIVE study. Neurology. 2020;95:e469–79.
Tepper SJ, Ashina M, Reuter U, Brandes JL, Doležil D, Silberstein SD, et al. Long-term safety and efficacy of erenumab in patients with chronic migraine: results from a 52-week, open-label extension study. Cephalalgia. 2020;40:543–53.
Ashina M, Kudrow D, Reuter U, Dolezil D, Silberstein S, Tepper SJ, et al. Long-term tolerability and nonvascular safety of erenumab, a novel calcitonin gene-related peptide receptor antagonist for prevention of migraine: a pooled analysis of four placebo-controlled trials with long-term extensions. Cephalalgia. 2019;39:1798–808.
Lattanzi S, Brigo F, Trinka E, Vernieri F, Corradetti T, Dobran M, et al. Erenumab for preventive treatment of migraine: a systematic review and meta-analysis of efficacy and safety. Drugs. 2019;79:417–31.
Zhu C, Guan J, Xiao H, Luo W, Tong R. Erenumab safety and efficacy in migraine: A systematic review and meta-analysis of randomized clinical trials. Medicine (Baltimore). 2019;98(52):e18483. https://doi.org/10.1097/MD.0000000000018483.
Sakaeda T, Tamon A, Kadoyama K, Okuno Y. Data mining of the public version of the FDA Adverse Event Reporting System. Int J Med Sci. 2013;10:796.
Zink RC, Huang Q, Zhang L-Y, Bao W-J. Statistical and graphical approaches for disproportionality analysis of spontaneously-reported adverse events in pharmacovigilance. Chin J Nat Med [Internet]. 2013;11:314–20.
Questions FDA. Answers on FDA’s Adverse Event Reporting System (FAERS). http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance.Advershtm. Accessed 3 Apr 2018 (WebCite Cache ID 6uhyyje6x).
Singh J. International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. J Pharmacol Pharmacother. 2015;6:185.
Kass-Hout TA, Xu Z, Mohebbi M, Nelsen H, Baker A, Levine J, et al. OpenFDA: an innovative platform providing access to a wealth of FDA’s publicly available data. J Am Med Inform Assoc. 2016;23:596–600.
European Medicine Agency. CHMP guideline on detection and management of duplicate individual cases and Individual Case Safety Reports (ICSRs) [Internet]. https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/chmp-guideline-detection-management-duplicate-individual-cases-individual-case-safety-reports_en.pdf. Accessed 11 Oct 2020.
Mascolo A, Scavone C, Sessa M, di Mauro G, Cimmaruta D, Orlando V, Rossi F, Sportiello L, Capuano A. Can causality assessment fulfill the new European definition of adverse drug reaction? A review of methods used in spontaneous reporting. Pharmacol Res. 2017;123:122–9. https://doi.org/10.1016/j.phrs.2017.07.005.
Sessa M, Rossi C, Mascolo A, Grassi E, Fiorentino S, Scavone C, et al. Suspected adverse reactions to contrast media in Campania Region (Italy): results from 14 years of post-marketing surveillance. Expert Opin Drug Saf [Internet]. 2015;14:1341–51.
Sessa M, Sportiello L, Mascolo A, Scavone C, Gallipoli S, di Mauro G, et al. Campania Preventability Assessment Committee (Italy): a focus on the preventability of non-steroidal anti-inflammatory drugs adverse drug reactions. Front Pharmacol Internet. https://doi.org/10.3389/fphar.2017.00305.
Sessa M, Sullo MG, Mascolo A, Cimmaruta D, Romano F, Puca RV, et al. A case of figurate urticaria by etanercept. J Pharmacol Pharmacother. 2016;7:106–8.
Sessa M, Rafaniello C, Sportiello L, Mascolo A, Scavone C, Maccariello A, et al. Campania Region (Italy) spontaneous reporting system and preventability assessment through a case-by-case approach: a pilot study on psychotropic drugs. Expert Opin Drug Saf. 2016;15:9–15.
Sessa M, di Mauro G, Mascolo A, Rafaniello C, Sportiello L, Scavone C, et al. Pillars and pitfalls of the new pharmacovigilance legislation: consequences for the identification of adverse drug reactions deriving from abuse, misuse, overdose, occupational exposure, and medication errors. Front Pharmacol; 2018;9:611.
Suen CY. n-Gram statistics for natural language understanding and text processing. IEEE Trans Pattern Anal Mach Intell. 1979;1(2):164–72. https://doi.org/10.1109/tpami.1979.4766902.
Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia. 2010;30:1065–72.
Minnema LA, Giezen TJ, Souverein PC, Egberts TCG, Leufkens HGM, Gardarsdottir H. Exploring the association between monoclonal antibodies and depression and suicidal ideation and behavior: a VigiBase Study. Drug Saf. 2019;42:887–95.
Essali N, Goldsmith DR, Carbone L, Miller BJ. Psychosis as an adverse effect of monoclonal antibody immunotherapy. Brain Behav Immun. 2019;81:646–9.
Daly TJ. Alopecia areata has low plasma levels of the vasodilator/immunomodulator calcitonin gene-related peptide. Arch. Dermatol. 1998;134(9):1164–5. https://doi.org/10.1001/archderm.134.9.1164.
Rossi R, Del Bianco E, Isolani D, Baccari MC, Cappugi P. Possible involvement of neuropeptidergic sensory nerves in alopecia areata. NeuroReport. 1997;8:1135–8.
Irimia P, Palma J-A, Idoate MA, España A, Riverol M, Martinez-Vila E. Cephalalgia alopecia or nummular headache with trophic changes? A new case with prolonged follow-up. Headache. 2013;53:994–7.
Zouboulis CC. Sebaceous gland receptors. Dermatoendocrinology. 2009;1:77–80.
Bigal ME, Liberman JN, Lipton RB. Age-dependent prevalence and clinical features of migraine. Neurology [Internet]. 2006;67:246 LP–251. http://n.neurology.org/content/67/2/246.abstract.
Collier CN, Harper JC, Cafardi JA, Cantrell WC, Wang W, Foster KW, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56–9.
Eftekhari S, Salvatore CA, Johansson S, Chen T-B, Zeng Z, Edvinsson L. Localization of CGRP, CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the blood-brain barrier. Brain Res. 2015;1600:93–109.
Kennis M, Gerritsen L, van Dalen M, Williams A, Cuijpers P, Bockting C. Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry [Internet]. 2020;25:321–38. https://doi.org/10.1038/s41380-019-0585-z.
Delvecchio G, Altamura AC, Soares JC, Brambilla P. Pituitary gland in bipolar disorder and major depression: evidence from structural mri studies: special section on “Translational and Neuroscience Studies in Affective Disorders”. Section Editor, Maria Nobile MD, PhD. This Section of JAD focuses on the r. J Affect Disord. 2017;218:446–50.
Zou Y, Xu F, Tang Z, Zhong T, Cao J, Guo Q, et al. Distinct calcitonin gene-related peptide expression pattern in primary afferents contribute to different neuropathic symptoms following chronic constriction or crush injuries to the rat sciatic nerve. Mol Pain [Internet]. 2016;12:1744806916681566–1744806916681566. https://pubmed.ncbi.nlm.nih.gov/28256957.
Nixdorf DR, Velly AM, Alonso AA. Neurovascular pains: implications of migraine for the oral and maxillofacial surgeon. Oral Maxillofac Surg Clin N Am [Internet]. 2008;20:221–vii. https://pubmed.ncbi.nlm.nih.gov/18343327.
Singh Y, Gupta G, Shrivastava B, Dahiya R, Tiwari J, Ashwathanarayana M, et al. Calcitonin gene-related peptide (CGRP): A novel target for Alzheimer’s disease. CNS Neurosci Ther. 2017;23:457–61.
Hou M, Xing H, Cai Y, Li B, Wang X, Li P, et al. The effect and safety of monoclonal antibodies to calcitonin gene-related peptide and its receptor on migraine: a systematic review and meta-analysis. J Headache Pain [Internet]. 2017;18:42. https://pubmed.ncbi.nlm.nih.gov/28389966.
Cha Y-H. Migraine-associated vertigo: diagnosis and treatment. Semin Neurol [Internet]. 2010;30:167–74. https://pubmed.ncbi.nlm.nih.gov/20352586.
Kong W-J, Scholtz AW, Kammen-Jolly K, Glückert R, Hussl B, von Cauvenberg PB, et al. Ultrastructural evaluation of calcitonin gene-related peptide immunoreactivity in the human cochlea and vestibular endorgans. Eur J Neurosci. 2002;15:487–97.
Marco RA, Hoffman LF, Wackym PA, Micevych PE, Popper P. Distribution of calcitonin gene-related peptide immunoreactivity in vestibular efferent neurons of the chinchilla. Hear Res. 1996;97:95–101.
Huang AY, Wu SY. Calcitonin gene-related peptide reduces taste-evoked ATP secretion from mouse taste buds. J Neurosci. 2015;35:12714–24.
Langguth B, Hund V, Busch V, Jürgens TP, Lainez J-M, Landgrebe M, et al. Tinnitus and Headache. Biomed Res Int [Internet]. 2015;2015:797416. https://pubmed.ncbi.nlm.nih.gov/26583133.
Hoshino T, Tabuchi K, Hara A. Effects of NSAIDs on the inner ear: possible involvement in cochlear protection. Pharmaceuticals (Basel). 2010;3:1286–95.
Chang AB, Gibson PG, Ardill J, McGarvey LPA. Calcitonin gene-related peptide relates to cough sensitivity in children with chronic cough. Eur Respir J. 2007;30:66–72.
Espinosa-Sanchez JM, Lopez-Escamez JA. New insights into pathophysiology of vestibular migraine. Front Neurol. 2015;6:12.
Kee Z, Kodji X, Brain SD. The role of calcitonin gene related peptide (CGRP) in neurogenic vasodilation and its cardioprotective effects. Front Physiol [Internet]. 2018;9:1249. https://pubmed.ncbi.nlm.nih.gov/30283343.
Kudrow D, Pascual J, Winner PK, Dodick DW, Tepper SJ, Reuter U, et al. Vascular safety of erenumab for migraine prevention. Neurology. 2020;94:e497-510.
Ashina M, Goadsby PJ, Reuter U, Silberstein S, Dodick D, Rippon GA, et al. Long-term safety and tolerability of erenumab: Three-plus year results from a five-year open-label extension study in episodic migraine. Cephalalgia. 2019;39:1455–64.
Ahima RS, Antwi DA. Brain regulation of appetite and satiety. Endocrinol Metab Clin N Am. 2008;37:811–23.
Austin J, Marks D. Hormonal regulators of appetite. Int J Pediatr Endocrinol [Internet]. 2009;2009:141753. https://pubmed.ncbi.nlm.nih.gov/19946401.
Ornello R, Ripa P, Pistoia F, Degan D, Tiseo C, Carolei A, et al. Migraine and body mass index categories: a systematic review and meta-analysis of observational studies. J Headache Pain [Internet]. 2015;16:27. https://pubmed.ncbi.nlm.nih.gov/25903159
Altomare A, Guarino MPL, Cocca S, Emerenziani S, Cicala M. Gastroesophageal reflux disease: update on inflammation and symptom perception. World J Gastroenterol [Internet]. 2013;19:6523–8. https://pubmed.ncbi.nlm.nih.gov/24151376.
T Noghani M, Rezaeizadeh H, Fazljoo SMB, Keshavarz M. Gastrointestinal Headache; a Narrative Review. Emerg (Tehran, Iran) [Internet]. 2016;4:171–83. https://pubmed.ncbi.nlm.nih.gov/27800536.
Tepper SJ, Stillman MJ. Clinical and preclinical rationale for CGRP-receptor antagonists in the treatment of migraine. Headache. 2008;48:1259–68.
Alomar M, Tawfiq AM, Hassan N, Palaian S. Post marketing surveillance of suspected adverse drug reactions through spontaneous reporting: current status, challenges and the future. Ther Adv drug Saf. 2020;11:2042098620938595.
Author information
Authors and Affiliations
Contributions
Drafting the work and revising it for important intellectual content: MS and MA. Substantial contributions to the acquisition, analysis, or interpretation of data for the work: MS. Final approval of the version to be published: MS and MA. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: MS and MA. Developed the concept and designed the study: MS. Wrote the paper: MS.
Corresponding author
Ethics declarations
Funding
MS and MA belong to the Pharmacovigilance Research Center, Department of Drug Design and Pharmacology, University of Copenhagen, supported by a grant from the Novo Nordisk Foundation (NNF15SA0018404).
Conflict of interest
Morten Andersen has during the past 5 years participated in research projects supported by grants from Novartis, Pfizer, Janssen, AstraZeneca, and H. Lundbeck & Mertz, received by the universities where he has been employed. He has personally received fees from Atrium Education, the Danish Pharmaceutical Industry Association, for teaching and leading pharmacoepidemiology courses. Maurizio Sessa has no conflicts of interest to declare.
Ethics approval
No ethical approval is required for descriptive studies using publicly available data from FAERS.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Code is available from the corresponding author on reasonable request.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sessa, M., Andersen, M. New Insight on the Safety of Erenumab: An Analysis of Spontaneous Reports of Adverse Events Recorded in the US Food and Drug Administration Adverse Event Reporting System Database. BioDrugs 35, 215–227 (2021). https://doi.org/10.1007/s40259-021-00469-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-021-00469-8