Abstract
Objective
The objective of this study was to compare the pharmacokinetics (PKs), safety, and immunogenicity of GB242 as a potential biosimilar infliximab with those of reference infliximab in healthy Chinese subjects.
Methods
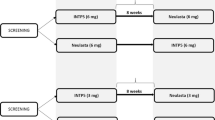
We conducted a randomized, single-center, double-blind, parallel-controlled phase I study in which 48 healthy subjects were divided equally into a GB242 group and reference infliximab group. Both the test and reference drug were administered as a single intravenous dose of 3 mg/kg. Blood samples were collected as per a designated schedule to evaluate PKs and immunogenicity. Safety was assessed throughout the study. PK similarity was concluded if the 90% confidence intervals (CIs) for the geometric mean ratios of the GB242 to reference infliximab for maximum concentration (Cmax), area under the concentration–time curve (AUC) from time zero to the last quantifiable concentration (AUCt), and AUC from time zero to infinity (AUC∞) were within the predefined bioequivalence range of 80–125%.
Results
The mean serum concentration–time curves were similar between GB242 and reference infliximab. The 90% CIs for the geometric mean ratios of the GB242 to reference infliximab for Cmax, AUCt, and AUC∞ were completely within 80–125% for the PK similarity comparison. The proportion of subjects with treatment-emergent adverse events was similar between the GB242 group and the reference infliximab group. Antidrug antibody profiles were comparable between the two treatments groups.
Conclusions
This study demonstrated high PK similarity between GB242 and its marketed reference infliximab in healthy subjects. Both treatments showed comparable safety and immunogenicity.
Registration number
ChiCTR-IPR-15007098


Similar content being viewed by others
References
Sanmartí R, Ruiz-Esquide V, Hernández MV. Rheumatoid arthritis: a clinical overview of new diagnostic and treatment approaches. Curr Top Med Chem. 2013;13(6):698–704.
Smolen JS, Aletaha DA, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38.
Cooles FA, Isaacs JD. Pathophysiology of rheumatoid arthritis. Curr Opin Rheumatol. 2011;23(3):233–40.
European Medicines Agency. Remicade, INN-infliximab. London: European Medicines Agency; 2005.
Putrik P, Ramiro S, Kvien TK, Sokka T, Pavlova M, Uhlig T, Boonen A. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann Rheum Dis. 2014;73:198–206.
China Food and Drug Administration. Guidelines for the development and evaluation of similar biological products. Beijing: China Food and Drug Administration; 2015.
European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. London: European Medicines Agency; 2014.
US Food and Drug Administration. Guidance for industry: scientific considerations in demonstrating biosimilarity to a reference product. Silver Spring: US Food and Drug Administration; 2012.
World Health Organization. Guidelines on evaluation of similar biotherapeutic products. Geneva: World Health Organization; 2009.
China Food and Drug Administration. Technical guidelines for the study of human bioavailability and bioequivalence in chemical pharmaceutical preparations. Beijing: China Food and Drug Administration; 2007.
World Medical Association. Declaration of Helsinki. Ethical principles for medical research involving human subjects. Ferney-Voltaire: World Medical Association; 2001.
China Food and Drug Administration. Guideline for good clinical principles. Beijing: China Food and Drug Administration; 2016.
US Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) version 4.0. J Natl Cancer Inst. 2009;09:5410.
Smith BP, Vandenhende FR, DeSante KA, Farid NA, Welch PA, Callaghan JT, et al. Confidence interval criteria for assessment of dose proportionality. Pharm Res. 2000;17(10):1278–83.
Wolbink GJ, Voskuyl AE, Lems WF, Groot ED, Nurmohamed MT, Tak PP, et al. Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2005;64:704–7.
Mori S. A relationship between pharmacokinetics (PK) and the efficacy of infliximab for patients with rheumatoid arthritis: characterization of infliximab-resistant cases and PK-based modified therapy. Mod Rheumatol. 2007;17:83–91.
US Food and Drug Administration. Clinical pharmacology review of BLA 99–0128. Remicade Supplement. Silver Spring: US Food and Drug Administration; 1999.
Shin D, Kim Y, Kim YS, Kornicke T, Fuhr R. A randomized, phase I pharmacokinetic study comparing SB2 and infliximab reference drug (Remicade®) in healthy subjects. BioDrugs. 2015;29(6):381–8.
Danesh A, Janghorbani M, Khalatbari S. Effects of antenatal corticosteroids on maternal serum indicators of infection in women at risk for preterm delivery: a randomized trial comparing betamethasone and dexamethasone. J Res Med Sci. 2012;17(10):911–7.
Schuld A, Birkmann S, Beitinger P, Haack M, Kraus T, Dalal MA, et al. Low doses of dexamethasone affect immune parameters in the absence of immunological stimulation. Exp Clin Endocrinol Diabetes. 2006;114(6):322–8.
Liles WC, Huang JE, Llewellyn C, SenGupta D, Price TH, Dale DC. A comparative trial of granulocyte-colony-stimulating factor and dexamethasone, separately and in combination, for the mobilization of neutrophils in the peripheral blood of normal volunteers. Transfusion. 1997;37(2):182–7.
Dexamethasone sodium phosphate. Product information. 2010. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2011-PI-02528-3. Accessed 20 Sep 2018.
Acknowledgements
Genor Biopharma Co. Ltd. (Shanghai, China) provided the test drugs (GB242 and reference infliximab). The authors would like to extend thanks to all enrolled participants, investigators, and people who contributed to this study.
Author information
Authors and Affiliations
Contributions
Yi Fang, Qingshan Zheng, Youzhong An, Jie Lv, Huiyang Cheng, and Shengbin Ren designed and monitored the whole study. Tan Zhang and Guihong Chen contributed to the data analysis and paper writing equally. Li’an Zu and Qian Wang were responsible for pre-treatment, drug administration, and blood sample collection in this study. Chang Liu, Qi Wang, and Yitong Wang contributed to blood sample collection and data analysis. Haifeng Song and Lihou Dong were in charge of determination of drug concentrations. All authors provided critical review and approved this manuscript. Yi Fang is the guarantor for the overall content.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Funding
This study was funded by Genor Biopharma Co. Ltd. (Shanghai, China).
Conflict of interest
Huiyang Cheng and Shengbin Ren are employees of Genor Biopharma Co. Ltd. (Shanghai, China). Tan Zhang, Guihong Chen, Chang Liu, Li’an Zu, Qi Wang, Yitong Wang, Jie Lv, Youzhong An, Lihou Dong, Qian Wang, Qingshan Zheng, Haifeng Song, and Yi Fang have no conflicts of interest to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, T., Chen, G., Liu, C. et al. A Phase I Study Comparing the Pharmacokinetics, Safety, and Immunogenicity of Proposed Biosimilar GB242 and Reference Infliximab in Healthy Subjects. BioDrugs 33, 93–100 (2019). https://doi.org/10.1007/s40259-018-0326-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-018-0326-x