Abstract
SB5 (Imraldi®) is a biosimilar of the reference anti-TNF monoclonal antibody adalimumab. It is approved for use in the following indications for which reference adalimumab is approved: rheumatoid arthritis (RA), juvenile idiopathic arthritis [polyarticular juvenile idiopathic arthritis (pJIA) and enthesitis-related arthritis (ERA)], axial spondyloarthritis [ankylosing spondylitis (AS) and non-radiographic axial spondyloarthritis (nr-axSpA)], psoriatic arthritis (PsA), psoriasis, pediatric plaque psoriasis, hidradenitis suppurativa (HS), Crohn’s disease, pediatric Crohn’s disease, ulcerative colitis (UC), and non-infectious uveitis. SB5 has similar physicochemical and pharmacodynamic properties to those of reference adalimumab, and the pharmacokinetic similarity of these agents has been shown in healthy volunteers and patients with RA. SB5 demonstrated clinical efficacy considered equivalent to that of reference adalimumab in patients with RA, and was generally well tolerated in this population. The safety and tolerability profile of SB5 was similar to that of reference adalimumab, as was the immunogenicity profile. Switching from reference adalimumab to SB5 had no impact in terms of efficacy, safety or immunogenicity. The role of reference adalimumab in the management of RA, pJIA, ERA, AS, nr-axSpA, PsA, psoriasis, pediatric plaque psoriasis, HS, Crohn’s disease, UC and non-infectious uveitis is well established and SB5 provides an effective biosimilar alternative for patients requiring adalimumab therapy.

Similar content being viewed by others
References
European Medicines Agency. Imraldi 40 mg solution for injection: summary of product characteristics. 2017. http://www.ema.europa.eu/. Accessed 4 July 2018.
Lee N, Kim M, Lee JAJ, et al. Biosimilarity between Humira and the biosimilar candidate SB5 in product quality [abstract no. 2495]. In: ACR/ARHP annual meeting; 2017.
European Medicines Agency. CHMP assessment report: imraldi (adalimumab biosimilar). 2017. http://www.ema.europa.eu/. Accessed 4 July 2018.
Shin D, Lee Y, Kim H, et al. A randomized phase I comparative pharmacokinetic study comparing SB5 with reference adalimumab in healthy volunteers. J Clin Pharm Ther. 2017;42(6):672–8.
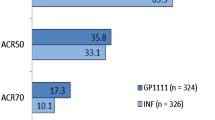
Weinblatt ME, Baranauskaite A, Niebrzydowski J, et al. Phase III randomized study of SB5, an adalimumab biosimilar, versus reference adalimumab in patients with moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2018;70(1):40–8.
Weinblatt ME, Baranauskaite A, Dokoupilova E, et al. Switching from reference adalimumab to SB5 (adalimumab biosimilar) in patients with rheumatoid arthritis: fifty-two-week phase III randomized study results. Arthritis Rheumatol. 2018;70(6):832–40.
Genovese M, Weinblatt M, Keystone E, et al. Impact of anti-drug antibodies on efficacy and safety up to week 24 from a phase III study comparing SB5 (an adalimumab biosimilar) with reference adalimumab in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy [abstract no. 48]. J Rheumatol. 2017;44(6):878.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. James Frampton is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: F. Araújo, Rheumatology and Osteoporosis Unit, Hospital Ortopédico de Sant’Ana, SCML, Lisbon, Portugal; S.R. Feldman, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, NC, USA; A. Sakuraba, Section of Gastroenterology, Hepatology and Nutrition, University of Chicago, Chicago, IL, USA.
Rights and permissions
About this article
Cite this article
Frampton, J.E. SB5: An Adalimumab Biosimilar. BioDrugs 32, 507–510 (2018). https://doi.org/10.1007/s40259-018-0307-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-018-0307-0