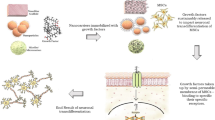
Abstract
Background
Neural injuries such as spinal cord injuries, traumatic brain injuries, or nerve transection injuries pose a major health problem. Neurotrophins such as nerve growth factor (NGF) or brain-derived neurotrophic factor (BDNF) have been shown to improve the outcome of neural injuries in several pre-clinical models, but their use in clinics is limited by the lack of a robust delivery system that enhances their bioavailability and half-life.
Objectives
We describe two fusion proteins comprising NGF or BDNF fused with elastin-like peptides (ELPs). The aim of this study was to investigate the biological activity of neurotrophin-ELP (N-ELP) fusion proteins via in vitro culture models.
Methods
NGF and BDNF were cloned in front of an elastin-like polypeptide sequence V40C2. These proteins were expressed in bacteria as inclusion bodies. These fusion proteins underwent solubilization via 8 M urea and purification via inverse transition cycling (ITC). We measured the particle size and the effect of temperature on precipitated particles using dynamic light scattering (DLS). We used western blot analysis to confirm the specificity of NGF-ELP to tropomyosin receptor kinase A (TrkA) antibody and to confirm the specificity of BDNF-ELP to TrkB antibody. PC12 cells were used to perform a neurite outgrowth assay to determine the biological activity of NGF-ELP. Bioactivity of BDNF-ELP was ascertained via transfecting human epithelial kidney (HEK 293-T) cells to express the TrkB receptor.
Results
The proteins were successfully purified to high homogeneity by exploiting the phase transition property of ELPs and urea, which solubilize inclusion bodies. Using PC12 neurite outgrowth assay, we further demonstrated that the biological activity of NGF was retained in the fusion. Similarly, BDNF-ELP phosphorylated the TrkB receptor, suggesting the biological activity of BDNF was also retained in the fusion. We further show that owing to the phase transition property of ELPs in the fusion, these proteins self-assembled into nanoparticles at their respective transition temperatures.
Conclusion
These fusion proteins are useful for neural regeneration, as they not only retain the biological activity of the neurotrophin but also self-assemble into nanoparticles, thereby simultaneously serving as drug-delivery vehicles. These nanoparticles can serve as drug depots and will increase bioavailability by limiting neurotrophin loss due to diffusion, thereby allowing controlled spatio-temporal delivery of the neurotrophin.







Similar content being viewed by others
References
Trumble TE, Shon FG. The physiology of nerve transplantation. Hand Clin. 2000;16(1):105–22.
Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49(6):377–91.
Blesch A, Tuszynski MH. Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 2009;32(1):41–7.
Willerth SM, Sakiyama-Elbert SE. Approaches to neural tissue engineering using scaffolds for drug delivery. Adv Drug Deliv Rev. 2007;59(4–5):325–38.
Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736.
Grill R, et al. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J Neurosci. 1997;17(14):5560–72.
Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76(2):99–125.
Mortazavi MM, et al. Chemical priming for spinal cord injury: a review of the literature part II-potential therapeutics. Childs Nerv Syst. 2011;27(8):1307–16.
Wang YC, et al. Sustained intraspinal delivery of neurotrophic factor encapsulated in biodegradable nanoparticles following contusive spinal cord injury. Biomaterials. 2008;29(34):4546–53.
Katz JS, Burdick JA. Hydrogel mediated delivery of trophic factors for neural repair. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(1):128–39.
Willerth SM, et al. Rationally designed peptides for controlled release of nerve growth factor from fibrin matrices. J Biomed Mater Res A. 2007;80(1):13–23.
Sly DJ, et al. Brain-derived neurotrophic factor modulates auditory function in the hearing cochlea. J Assoc Res Otolaryngol. 2012;13(1):1–16.
Lindsey S, et al. Beta hairpin peptide hydrogels as an injectable solid vehicle for neurotrophic growth factor delivery. Biomacromolecules. 2015:16(9):2672–83.
Wolfe D, Mata M, Fink DJ. Targeted drug delivery to the peripheral nervous system using gene therapy. Neurosci Lett. 2012;527(2):85–9.
Hirsch T, et al. Gene therapy in cutaneous wound healing. Front Biosci. 2007;12:2507–18.
Byrnes CK, et al. Success and limitations of a naked plasmid transfection protocol for keratinocyte growth factor-1 to enhance cutaneous wound healing. Wound Repair Regen. 2001;9(5):341–6.
Yao F, Eriksson E. Gene therapy in wound repair and regeneration. Wound Repair Regen. 2000;8(6):443–51.
Meyer DE, Chilkoti A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat Biotechnol. 1999;17(11):1112–5.
George EM, et al. Growth factor purification and delivery systems (PADS) for therapeutic angiogenesis. Vasc Cell. 2015;7(1):1.
Koria P, et al. Self-assembling elastin-like peptides growth factor chimeric nanoparticles for the treatment of chronic wounds. Proc Natl Acad Sci USA. 2011;108(3):1034–9.
Asai D, et al. Protein polymer hydrogels by in situ, rapid and reversible self-gelation. Biomaterials. 2012;33(21):5451–8.
Mie M, Mizushima Y, Kobatake E. Novel extracellular matrix for cell sheet recovery using genetically engineered elastin-like protein. J Biomed Mater Res Part B: Appl Biomater. 2008;86B(1):283–90.
Le DHT, Okubo T, Sugawara-Narutaki A. Beaded nanofibers assembled from double-hydrophobic elastin-like block polypeptides: effects of trifluoroethanol. Biopolymers. 2015;103(3):175–85.
Meyer DE, Chilkoti A. Genetically encoded synthesis of protein-based polymers with precisely specified molecular weight and sequence by recursive directional ligation: examples from the elastin-like polypeptide system. Biomacromolecules. 2002;3(2):357–67.
Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73(7):2424–8.
Soppet D, et al. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell. 1991;65(5):895–903.
Mohtaram NK, Montgomery A, Willerth SM. Biomaterial-based drug delivery systems for the controlled release of neurotrophic factors. Biomed Mater. 2013;8(2):022001.
Angelova A, et al. Neurotrophin delivery using nanotechnology. Drug Discov Today. 2013;18(23–24):1263–71.
Wang TY, et al. Biofunctionalisation of polymeric scaffolds for neural tissue engineering. J Biomater Appl. 2012;27(4):369–90.
Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J Control Release. 2000;69(1):149–58.
Sakiyama-Elbert SE, Panitch A, Hubbell JA. Development of growth factor fusion proteins for cell-triggered drug delivery. FASEB J. 2001;15(7):1300–2.
Sun W, et al. The effect of collagen-binding NGF-beta on the promotion of sciatic nerve regeneration in a rat sciatic nerve crush injury model. Biomaterials. 2009;30(27):4649–56.
Angelova A, et al. Protein entrapment in PEGylated lipid nanoparticles. Int J Pharm. 2013;454(2):625–32.
Dicou E. Expression of recombinant human nerve growth factor in Escherichia coli. Neurochem Int. 1992;20(1):129–34.
Negro A, et al. Synthesis and purification of biologically active rat brain-derived neurotrophic factor from Escherichia coli. Biochem Biophys Res Commun. 1992;186(3):1553–9.
Park E, et al. Production and characterization of fusion proteins containing transferrin and nerve growth factor. J Drug Target. 1998;6(1):53–64.
Meyer SL, et al. Production and characterization of recombinant mouse brain-derived neurotrophic factor and rat neurotrophin-3 expressed in insect cells. J Neurochem. 1994;62(3):825–33.
Rattenholl A, et al. The pro-sequence facilitates folding of human nerve growth factor from Escherichia coli inclusion bodies. Eur J Biochem. 2001;268(11):3296–303.
Bidwell GL, Raucher D. Cell penetrating elastin-like polypeptides for therapeutic peptide delivery. Adv Drug Deliv Rev. 2010;62(15):1486–96.
Iglesias R, Koria P. Leveraging growth factor induced macropinocytosis for targeted treatment of lung cancer. Med Oncol. 2015;32(12):259.
Ratner N, Bunge RP, Glaser L. A neuronal cell surface heparan sulfate proteoglycan is required for dorsal root ganglion neuron stimulation of Schwann cell proliferation. J Cell Biol. 1985;101(3):744–54.
Snow AD, et al. Early accumulation of heparan sulfate in neurons and in the beta-amyloid protein-containing lesions of Alzheimer’s disease and Down’s syndrome. Am J Pathol. 1990;137(5):1253–70.
Sandow SL, et al. Signalling organelle for retrograde axonal transport of internalized neurotrophins from the nerve terminal. Immunol Cell Biol. 2000;78(4):430–5.
Delcroix J-D, et al. NGF signaling in sensory neurons. Neuron. 2003;39(1):69–84.
Cheng PL, et al. Self-amplifying autocrine actions of BDNF in axon development. Proc Natl Acad Sci. 2011;108(45):18430–5.
Shao Y. Pincher, a pinocytic chaperone for nerve growth factor/TrkA signaling endosomes. J Cell Biol. 2002;157(4):679–91.
Valdez G. Pincher-mediated macroendocytosis underlies retrograde signaling by neurotrophin receptors. J Neurosci. 2005;25(21):5236–47.
Howe CL, et al. NGF signaling from clathrin-coated vesicles. Neuron. 2001;32(5):801–14.
Acknowledgments
The authors acknowledge Yuan Yuan, Raul Iglesias, and Bernard Batson.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Tamina Johnson and Piyush Koria have no conflicts of interest that are directly relevant to the content of this study.
Funding
This work was funded by startup funds from the University of South Florida (PK) and the new researcher grant (PK) from University of South Florida, and in part by Institutional Research Grant number 93-032-16 from the American Cancer Society (PK). Tamina Johnson was supported by NSF Florida Georgia Louis Stokes Alliance for Minority Participation Bridge to the Doctorate Award HRD #1139850, the USF Graduate Student Success Diversity Fellowship, and The Alfred P. Sloan Minority Ph.D. Program.
Rights and permissions
About this article
Cite this article
Johnson, T., Koria, P. Expression and Purification of Neurotrophin-Elastin-Like Peptide Fusion Proteins for Neural Regeneration. BioDrugs 30, 117–127 (2016). https://doi.org/10.1007/s40259-016-0159-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-016-0159-4