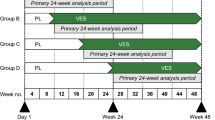
Abstract
Elosulfase alfa (Vimizim®) is a recombinant form of the human lysosomal enzyme N-acetylgalactosamine-6-sulfatase (GALNS) that is lacking in patients with mucopolysaccharidosis type IVA (MPS IVA; Morquio A syndrome). It is the first, and currently only, disease-specific treatment option for this very rare, progressively degenerative, autosomal-recessive lysosomal storage disorder. Enzyme replacement therapy with elosulfase alfa aims to restore GALNS activity, thereby preventing the accumulation of keratan sulfate (KS) and chondroitin-6-sulfate in lysosomal compartments of cells that results in the clinical manifestations of MPS IVA. In clinical trials in children and adults with MPS IVA, intravenous elosulfase alfa 2 mg/kg/week provided significant and sustained improvements in urinary levels of KS (a pharmacodynamic biomarker for the disease). In the key placebo-controlled, 24-week, phase 3 trial in patients with MPS IVA aged ≥5 years, elosulfase alfa 2 mg/kg/week significantly improved endurance [least squares mean placebo-adjusted change from baseline in 6-min walk test distance 22.5 m (95 % CI 4.0–40.9)]. Infusion-associated reactions, the primary tolerability issue associated with elosulfase alfa, are generally mild to moderate in severity, self-limiting, and manageable. In the absence of a cure, GALNS enzyme replacement therapy with elosulfase alfa is an important achievement in the treatment of MPS IVA.

Similar content being viewed by others
References
Montaño AM, Tomatsu S, Gottesman GS, et al. International Morquio A Registry: clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis. 2007;30(2):165–74.
Harmatz P, Mengel KE, Giugliani R, et al. The Morquio A Clinical Assessment Program: baseline results illustrating progressive, multisystemic clinical impairments in Morquio A subjects. Mol Genet Metab. 2013;109(1):54–61.
Hendriksz CJ, Harmatz P, Beck M, et al. Review of clinical presentation and diagnosis of mucopolysaccharidosis IVA. Mol Genet Metab. 2013;110(1–2):54–64.
Wood TC, Harvey K, Beck M, et al. Diagnosing mucopolysaccharidosis IVA. J Inherit Metab Dis. 2013;36(2):293–307.
Nelson J, Crowhurst J, Carey B, et al. Incidence of the mucopolysaccharidoses in Western Australia. Am J Med Genet A. 2003;123A(3):310–3.
Nelson J. Incidence of the mucopolysaccharidoses in Northern Ireland. Hum Genet. 1997;101(3):355–8.
Baehner F, Schmiedeskamp C, Krummenauer F, et al. Cumulative incidence rates of the mucopolysaccharidoses in Germany. J Inherit Metab Dis. 2005;28(6):1011–7.
Lin HY, Lin SP, Chuang CK, et al. Incidence of the mucopolysaccharidoses in Taiwan, 1984–2004. Am J Med Genet A. 2009;149A(5):960–4.
Ben Turkia H, Tebib N, Azzouz H, et al. Incidence of mucopolysaccharidoses in Tunisia. Tunis Med. 2009;87(11):782–5.
Applegarth DA, Toone JR, Lowry RB. Incidence of inborn errors of metabolism in British Columbia, 1969–1996. Pediatrics. 2000;105(1):e10.
Poorthuis BJ, Wevers RA, Kleijer WJ, et al. The frequency of lysosomal storage diseases in The Netherlands. Hum Genet. 1999;105(1–2):151–6.
Pinto R, Caseiro C, Lemos M, et al. Prevalence of lysosomal storage diseases in Portugal. Eur J Hum Genet. 2004;12(2):87–92.
Lachman RS, Burton BK, Clarke LA, et al. Mucopolysaccharidosis IVA (Morquio A syndrome) and VI (Maroteaux–Lamy syndrome): under-recognized and challenging to diagnose. Skeletal Radiol. 2014;43:359–69.
Tomatsu S, Montaño AM, Nishioka T, et al. Mutation and polymorphism spectrum of the GALNS gene in mucopolysaccharidosis IVA (Morquio A). Hum Mutat. 2005;26(6):500–12.
Rivera-Colon Y, Schutsky EK, Kita AZ, Garman SC. The structure of human GALNS reveals the molecular basis for mucopolysaccharidosis IV A. J Mol Biol. 2012;423(5):736–51.
Morrone A, Tylee KL, Al-Sayed M, et al. Molecular testing of 163 patients with Morquio A (Mucopolysaccharidosis IVA) identifies 39 novel GALNS mutations. Mol Genet Metab. 2014;112(2):160–70.
Algahim MF, Almassi GH. Current and emerging management options for patients with Morquio A syndrome. Ther Clin Risk Manag. 2013;9(1):45–53.
Solanki GA, Martin KW, Theroux MC, et al. Spinal involvement in mucopolysaccharidosis IVA (Morquio-Brailsford or Morquio A syndrome): presentation, diagnosis and management. J Inherit Metab Dis. 2013;36(2):339–55.
White KK, Jester A, Bache CE, et al. Orthopedic management of the extremities in patients with Morquio A syndrome. J Child Orthop. 2014;8(4):295–304.
Hendriksz CJ, Al-Jawad M, Berger KI, et al. Clinical overview and treatment options for non-skeletal manifestations of mucopolysaccharidosis type IVA. J Inherit Metab Dis. 2013;36(2):309–22.
Braunlin EA, Harmatz PR, Scarpa M, et al. Cardiac disease in patients with mucopolysaccharidosis: presentation, diagnosis and management. J Inherit Metab Dis. 2011;34(6):1183–97.
Tomatsu S, Montano AM, Oikawa H, et al. Mucopolysaccharidosis type IVA (Morquio A disease): clinical review and current treatment. Curr Pharm Biotechnol. 2011;12(6):931–45.
Hendriksz CJ, Lavery C, Coker M, et al. Burden of disease in patients with Morquio A syndrome: results from an international patient-reported outcomes survey. Orphanet J Rare Dis. 2014;9:32. doi:10.1186/1750-1172-9-32.
Davison JE, Kearney S, Horton J, et al. Intellectual and neurological functioning in Morquio syndrome (MPS IVa). J Inherit Metab Dis. 2013;36(2):323–8.
Lavery C, Hendriksz C. Mortality in patients with Morquio syndrome A. JIMD Rep. [Epub 10 Apr 2014].
Hendriksz CJ, Burton B, Fleming TR, et al. Efficacy and safety of enzyme replacement therapy with BMN 110 (elosulfase alfa) for Morquio A syndrome (mucopolysaccharidosis IVA): a phase 3 randomised placebo-controlled study. J Inherit Metab Dis. 2104. doi:10.1007/s10545-014-9715-6 [Epub 9 May 2014].
Hendriksz CJ, Vellodi A, Jones S, et al. A multicenter, open-label, extension study to evaluate the long-term efficacy and safety of BMN110 in patients with mucopolysaccharidosis IVA (MPS IVA, Morquio A syndrome) [abstract no. P-733]. J Inherit Metab Dis. 2013;36(Suppl 2):S303.
Qi Y, Musson D, Martell L, et al. Phase 1/2 pharmacokinetic studies of recombinant human N-acetylgalactosamine-6-sulfatase (rhGALNS) in MPS IVA patients [abstract]. In: American Association of Pharmaceutical Scientists Annual Meeting; 14–18 Oct 2012; Chicago (IL).
Qi Y, Musson D, Tompkins T, et al. Pharmacokinetic and pharmacodynamic evaluation of elosulfase alfa, an enzyme replacement therapy in patients with Morquio A syndrome: results from MOR-004, a phase 3 trial [abstract no. 76]. In: American College of Medical Genetics 2014 Annual Clinical Genetics Meeting; 25–29 Mar 2014; Nashville (TN).
Lorget F, Lee S, Lau K, et al. BMN 110 (recombinant human GALNS), an investigational enzyme replacement therapy for mucopolysaccharidosis type iva (MPS IVA or morquio syndrome), promotes type II collagen synthesis in MPS IVA patients [abstract]. Mol Genet Metab. 2012;105(2):S44.
Harmatz P, Jones S, Bialer M, et al. Safety and pharmacodynamic activity of elosulfase alfa in pediatric patients less than 5 years of age with Morquio A syndrome (mucopolysaccharidosis IVA) [abstract]. In: 13th International Symposium on Mucopolysaccaridoses and Related Diseases; 13–17 Aug 2014; Costa do Sauipe, Brazil.
Vimizim (elosulfase alfa) 1 mg/mL concentrate for solution for infusion: summary of product characteristics. London: European Medicines Agency; 2014.
Vimizim (elosulfase alfa) injection, for intravenous use: US prescribing information. Novato (CA): Biomarin Pharmaceutical Inc.; 2014.
Dvorak-Ewell M, Wendt D, Hague C, et al. Enzyme replacement in a human model of mucopolysaccharidosis IVA in vitro and its biodistribution in the cartilage of wild type mice. PLoS One. 2010;5(8):e12194. doi:10.1371/journal.pone.0012194.
Harmatz P, Hendriksz CJ, Giugliani R, et al. Long term safety analysis of BMN110 dosed at 2 mg/kg/week in 52 subjects with mucopolysaccharidosis (Morquio A syndrome, MPSIVA) [abstract no. P-732]. J Inherit Metab Dis. 2013;36(Suppl 2): S303. Plus poster presented at the 12th International Congress of Inborn Errors of Metabolism; 3–6 Sep 2013; Barcelona.
Edano C, Malick M, Burton B. Infusion management of elosulfase alfa for patients with Morquio A syndrome [abstract]. In: 13th International Symposium on Mucopolysaccharidoses and Related Diseases; 13–17 Aug 2014; Costa Do Sauipe, Brazil.
McDonald CM, Henricson EK, Abresch RT, et al. The 6-minute walk test and other clinical endpoints in duchenne muscular dystrophy: reliability, concurrent validity, and minimal clinically important differences from a multicenter study. Muscle Nerve. 2013;48(3):357–68.
Shoemaker MK, Curtis AB, Vangsnes E, et al. Clinically meaningful change estimates for the six-minute walk test and daily activity in individuals with chronic heart failure. Cardiopulm Phys Ther J. 2013;24(3):21–9.
Mathai SC, Puhan MA, Lam D, et al. The minimal important difference in the 6-minute walk test for patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186(5):428–33.
Swigris JJ, Wamboldt FS, Behr J, et al. The six-minute walk in idiopathic pulmonary fibrosis: longitudinal changes and minimum important difference. Thorax. 2010;65(2):173–7.
Henricson E, Abresch R, Han JJ, et al. The 6-minute walk test and person-reported outcomes in boys with Duchenne muscular dystrophy and typically developing controls: longitudinal comparisons and clinically-meaningful changes over one year. PLoS Curr. 2013;5. doi: 10.1371/currents.md.9e17658b007eb79fcd6f723089f79e06.
Graham S, Waite A, Loba-Olopade O, et al. The Morquio A Registry Study (MARS): improving the understanding of Morquio A syndrome [abstract]. In: Annual Society for the Study of Inborn Errors of Metabolism Symposium; 2–5 Sep 2014; Innsbruck.
Disclosure
The preparation of this review was not supported by any external funding. K.A. Lyseng-Williamson is a salaried employee of Adis/Springer. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author(s) on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: M. Beck, Children’s Hospital, University of Mainz, Mainz, Germany; D. Hughes, Royal and Free University College Medical School, London, UK; E. Mengel, Villa Metabolica, Centre for Pediatric and Adolescent Medicine, Medical Center University of Mainz, Mainz, Germany; J. Mitchell, Department of Medical Genetics, McGill University Health Centre, Montreal, Quebec, Canada.
Rights and permissions
About this article
Cite this article
Lyseng-Williamson, K.A. Elosulfase Alfa: A Review of Its Use in Patients with Mucopolysaccharidosis Type IVA (Morquio A Syndrome). BioDrugs 28, 465–475 (2014). https://doi.org/10.1007/s40259-014-0108-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-014-0108-z