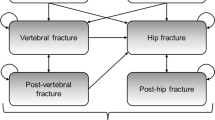
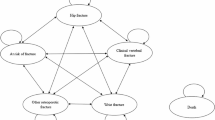
Abstract
Objective
This study evaluated the cost effectiveness of denosumab versus alendronate for secondary prevention of osteoporotic fractures among post-menopausal women in China.
Methods
A validated individual-level simulation model of osteoporotic fractures in the Chinese setting was adapted. Allowing both treatment discontinuation and waning effects, the analysis aimed to evaluate the incremental cost-effectiveness ratio of denosumab compared to alendronate by simulating a cohort of previously fractured individuals over the residual lifetime from the healthcare system perspective. Hip, vertebral, and wrist/humeral fractures were tracked along with the associated medical costs and quality-adjusted life-years. Age-related health state utility values, health state utility values of fractures, costs, fracture incidence, and mortality risks for Chinese were used whenever available. Comparative effectiveness data were obtained from a published network meta-analysis. One-way and probabilistic sensitivity analyses were conducted.
Results
In the base case, denosumab was dominated by alendronate with incremental costs of CN¥2743 (US$425) and incremental health outcomes of − 0.20 quality-adjusted life-years at its current price in mainland China. It remained dominated in all one-way sensitivity analysis robustness checks. However, denosumab was cost effective if both drugs did not carry any waning effects. In the probabilistic sensitivity analysis, denosumab remained dominated in all replications.
Conclusions
Denosumab is not cost effective for preventing secondary fractures among overall postmenopausal women in China. It is advisable to identify alternative denosumab regimens for high-risk subgroups among previously fractured postmenopausal women.



Similar content being viewed by others
References
Kanis JA, Black D, Cooper C, et al. A new approach to the development of assessment guidelines for osteoporosis. Osteoporos Int. 2002;13(7):527–36.
Bimal G, Sahhar J, Savanur M, et al. Screening rates and prevalence of osteoporosis in a real-world. Austral Syst Scler cohort. 2022;25(2):175–81.
Osteoporosis: review of the evidence for prevention, diagnosis and treatment and cost-effectiveness analysis. Introduction. Osteoporos Int. 1998;8 Suppl. 4:S7–80. https://pubmed.ncbi.nlm.nih.gov/10197173/.
Jiang Y, Ni W. Expected lifetime numbers, risks, and burden of osteoporotic fractures for 50-year old Chinese women: a discrete event simulation incorporating FRAX. J Bone Miner Metab. 2015;18:A643.
Si L, Winzenberg TM, Chen M, et al. Residual lifetime and 10 year absolute risks of osteoporotic fractures in Chinese men and women. Curr Med Res Opin. 2015;31(6):1149–56.
Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359(9321):1929–36.
Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359(9319):1761–7.
Liang H, Zhong W, Ning L. Statistics on the expenses for medical care of osteoporotic fractures in Beijing Jishuitan Hospital (from 2000 to 2006). J Pract Orthopaed. 2009;5:1–25.
Wu J, Qu Y, Wang K, et al. Healthcare resource utilization and direct medical costs for patients with osteoporotic fractures in China. Value Health Reg Issues. 2019;18:106–11.
Kanis JA, Oden A, Johnell O, et al. The components of excess mortality after hip fracture. Bone. 2003;32(5):468–73.
Hasegawa Y, Suzuki S, Wingstrand H. Risk of mortality following hip fracture in Japan. J Orthop Sci. 2007;12(2):113–7.
Iolascon G, Moretti A, Toro G, et al. Pharmacological therapy of osteoporosis: what’s new? Clin Interv Aging. 2020;15:485–91.
Appelman-Dijkstra NM, Oei HLDW, Vlug AG, et al. The effect of osteoporosis treatment on bone mass. Best Pract Res Clin Endocrinol Metab. 2022;36(2):101623.
Pedersen AB, Heide-Jørgensen U, Sørensen HT, et al. Comparison of risk of osteoporotic fracture in denosumab vs alendronate treatment within 3 years of initiation. JAMA Netw Open. 2019;2(4):e192416.
Chandran T, Venkatachalam I. Efficacy and safety of denosumab compared to bisphosphonates in improving bone strength in postmenopausal osteoporosis: a systematic review. Singapore Med J. 2019;60(7):364–78.
Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis; 2020 update. Endocr Pract. 2020;26:1–46.
Kanis JA, Cooper C, Rizzoli R, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30(1):3–44.
Storm NE, Chang W, Lin TC, et al. A novel case study of the use of real-world evidence to support the registration of an osteoporosis product in China. Ther Innov Regul Sci. 2022;56(1):137–44.
Ni W, Jiang Y. Evaluation on the cost-effective threshold of osteoporosis treatment on elderly women in China using discrete event simulation model. Osteoporos Int. 2017;28(2):529–38.
Caro JJ, Möller J, Karnon J, et al. Discrete event simulation for health technology assessment. New York: CRC Press; 2015.
Caro JJ, Möller J. Advantages and disadvantages of discrete-event simulation for health economic analyses. Expert Rev Pharmacoecon Outcomes Res. 2016;16(3):327–9.
Bleibler F, Rapp K, Jaensch A, et al. Expected lifetime numbers and costs of fractures in postmenopausal women with and without osteoporosis in Germany: a discrete event simulation model. BMC Health Serv Res. 2014;14:284.
Caro JJ. Pharmacoeconomic analyses using discrete event simulation. Pharmacoeconomics. 2005;23(4):323–32.
World Health Organization. Life tables by country (GHE: life tables). 2019. https://www.who.int/data/gho/data/indicators/indicator-details/GHO/gho-ghe-life-tables-by-country. Accessed 20 Oct 2020.
Hayes KN, Winter EM, Cadarette SM, et al. Duration of bisphosphonate drug holidays in osteoporosis patients: a narrative review of the evidence and considerations for decision-making. J Clin Med. 2021;10(5):1140.
Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMJ (Clin Res Ed). 2022;376:e067975.
Wang L, Yu W, Yin X, et al. Prevalence of osteoporosis and fracture in China: the China Osteoporosis Prevalence Study. JAMA Netw Open. 2021;4(8):e2121106.
Si L, Winzenberg TM, Palmer AJ. A systematic review of models used in cost-effectiveness analyses of preventing osteoporotic fractures. Osteoporos Int. 2014;25(1):51–60.
Kung AW, Lee KK, Ho AY, et al. Ten-year risk of osteoporotic fractures in postmenopausal Chinese women according to clinical risk factors and BMD T-scores: a prospective study. J Bone Miner Res. 2007;22(7):1080–7.
Bow CH, Cheung E, Cheung CL, et al. Ethnic difference of clinical vertebral fracture risk. Osteoporos Int. 2012;23(3):879–85.
Gidwani R, Russell LB. Estimating transition probabilities from published evidence: a tutorial for decision modelers. Pharmacoeconomics. 2020;38(11):1153–64.
Ettinger B, Black D, Dawson-Hughes B, et al. Updated fracture incidence rates for the US version of FRAX®. Osteoporos Int. 2010;21(1):25–33.
Yoshizawa T, Nishino T, Okubo I, et al. Cost-effectiveness analysis of drugs for osteoporosis treatment in elderly Japanese women at high risk of fragility fractures: comparison of denosumab and weekly alendronate. Arch Osteoporos. 2018;13(1):94.
Mori T, Crandall CJ, Fujii T, et al. Cost-effectiveness of zoledronic acid compared with sequential denosumab/alendronate for older osteoporotic women in Japan. Arch Osteoporos. 2021;16(1):113.
Freemantle N, Cooper C, Diez-Perez A, et al. Results of indirect and mixed treatment comparison of fracture efficacy for osteoporosis treatments: a meta-analysis. Osteoporos Int. 2013;24(1):209–17.
Boschitsch E, Naegele O, Klinger A, et al. Long-term persistence with denosumab: real-world data from the Austrian Osteoporosis Clinic (AOC). A retrospective data analysis Osteoporos Int. 2022;33(1):263–72.
Lo JC, Pressman AR, Omar MA, et al. Persistence with weekly alendronate therapy among postmenopausal women. Osteoporos Int. 2006;17(6):922–8.
Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res. 2018;33(2):190–8.
Tripto-Shkolnik L, Fund N, Rouach V, et al. Fracture incidence after denosumab discontinuation: real-world data from a large healthcare provider. Bone. 2020;130:115150.
Mori T, Crandall CJ, Ganz DA. Cost-effectiveness of denosumab versus oral alendronate for elderly osteoporotic women in Japan. Osteoporos Int. 2017;28(5):1733–44.
Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301(5):513–21.
yaozh.com. Information of drug winning bid. 2021. https://data.yaozh.com/yaopinzhongbiao. Accessed 17 Jun 2021.
You R, Liu J, Ke L, et al. Cost-effectiveness of sequential denosumab/zoledronic acid compared with zoledronic acid monotherapy for postmenopausal osteoporotic women in China. Front Pharmacol. 2022;13:816248.
National Health Commission of China. Statistics bulletin of china health development 2020 (in Chinese). 2021. http://www.gov.cn/guoqing/2021-07/22/content_5626526.htm. Accessed 2 Aug 2021.
XE currency table: USD - US dollar. 2020. https://www.xe.com/currencycharts/?from=USD&to=CNY&view=5Y. Accessed 10 Oct 2021.
Si L, Winzenberg TM, de Graaff B, et al. A systematic review and meta-analysis of utility-based quality of life for osteoporosis-related conditions. Osteoporos Int. 2014;25(8):1987–97.
Si L, Shi L, Chen M, et al. Establishing benchmark EQ-5D-3L population health state utilities and identifying their correlates in Gansu Province, China. Qual Life Res. 2017;26(11):3049–58.
Liu G, Wu J, Sun L, et al. China guidelines for pharmacoeconomic evaluations 2019. Beijing: Chinese Pharmaceutical Association; 2019.
Cai D, Shi S, Jiang S, et al. Estimation of the cost-effective threshold of a quality-adjusted life year in China based on the value of statistical life. Eur J Health Econ. 2021;23:607–15.
National Bureau of Statistics of China. Statistical communiqué of the People's Republic of China on the 2021 national economic and social development. 2022. http://www.stats.gov.cn/english/PressRelease/202202/t20220227_1827963.html. Accessed 2 Apr 2022.
Center JR, Bliuc D, Nguyen TV, et al. Risk of subsequent fracture after low-trauma fracture in men and women. JAMA. 2007;297(4):387–94.
You R, Mori T, Ke L, et al. Which injected antiosteoporotic medication is worth paying for? A cost-effectiveness analysis of teriparatide, zoledronate, ibandronate, and denosumab for postmenopausal osteoporotic women in China. Menopause. 2022;29(2):210–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This project was funded in part by the National Natural Science Foundation of China (Grant No. 72004242). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Conflicts of Interest/Competing Interests
The authors have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
The data analyzed during the study are presented in the article and its supplementary materials.
Code Availability
The model used in this study was provided to the journal’s peer reviewers for their reference when reviewing the manuscript and is available from the lead author and the corresponding author upon reasonable requests.
Authors’ Contributions
YJ and LS had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: YJ and LS. Acquisition of data: YJ, SJ, SS, ML, and LS. Drafting of the manuscript: YJ and SJ. Critical revision of the manuscript for important intellectual content: LL, SS, ML, and LS. Modeling: YJ and LLi. Analysis and interpretation: YJ and LL. Obtained funding: YJ. Administrative, technical, or material support: SJ, SS, and LS.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, Y., Jiang, S., Li, L. et al. Cost Effectiveness of Denosumab for Secondary Prevention of Osteoporotic Fractures Among Postmenopausal Women in China: An Individual-Level Simulation Analysis. Appl Health Econ Health Policy 21, 489–499 (2023). https://doi.org/10.1007/s40258-022-00784-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-022-00784-3