Abstract
Background
Despite worldwide use of parenteral methotrexate (pMTX), health economic evidence for its use in Crohn’s disease (CD) is limited. The low price of this generic drug has removed any commercial incentive to further invest in research. However, there is an unmet need for treatment of mild-to-moderate CD, since biological/targeted therapies are usually reserved for patients with more severe disease due to the higher costs of these treatments.
Objective
To evaluate the cost-effectiveness of pMTX compared to the standard of care (SOC, i.e., high doses of oral corticosteroids (hdCS) followed by gradual tapering) for the treatment of mild-to-moderate CD in the Czech Republic.
Methods
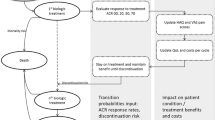
We developed a 3-year Markov model with a 1-week cycle length comprising five health states. The model projected quality-adjusted life-years (QALYs) and costs from the healthcare payers’ perspective. Efficacy data were obtained from a systematic literature review of clinical trials and extrapolated using survival analysis.
Results
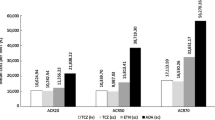
Over a 3-year time-horizon, pMTX yields additional 0.111 QALYs (1.798 vs. 1.687) at an additional cost of €513 (€3087 vs. €2574), with an incremental deterministic (probabilistic) cost-effectiveness ratio of €4627 (€4742)/QALY, far below the willingness-to-pay (WTP) threshold (≈ €47,000/QALY). The probabilistic sensitivity analysis showed that the probability of pMTX being cost-effective was 100%. A one-way sensitivity and scenario analysis confirmed the robustness of the base-case result.
Conclusion
Parenteral MTX proved to be cost-effective in patients with mild-to-moderate CD. This is the first published cost-effectiveness analysis of pMTX for this indication. It also shows an example of a lack of valuation of generic therapy despite its cost-effectiveness and a clear benefit to the healthcare system.





Similar content being viewed by others
References
Feagan BG, Rochon J, Fedorak RN, Irvine EJ, Wild G, Sutherland L, et al. Methotrexate for the treatment of Crohn’s disease. The North American Crohn’s Study Group Investigators. N Engl J Med. 1995;332:292–7.
Chebli JMF, Gaburri PD, De Souza AFM, Pinto ALT, Chebli LA, Felga GEG, et al. Long-term results with azathioprine therapy in patients with corticosteroid-dependent Crohn’s disease: open-label prospective study. J Gastroenterol Hepatol. 2007;22:268–74.
Prefontaine E, Sutherland LR, Macdonald JK, Cepoiu M. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2009;20:CD000067.
Patel V, Wang Y, MacDonald JK, McDonald JWD, Chande N. Methotrexate for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2014;20:CD006884.
Ward MM. Trends in the use of disease modifying antirheumatic medications in rheumatoid arthritis, 1980–1995: results from the National Ambulatory Medical Care Surveys. J Rheumatol. 1999;26:546–50.
Maetzel A, Bombardier C, Strand V, Tugwell P, Wells G. How Canadian and US rheumatologists treat moderate or aggressive rheumatoid arthritis: a survey. J Rheumatol. 1998;25:2331–8.
Willkens RF, Sharp JT, Stablein D, Marks C, Wortmann R. Comparison of azathioprine, methotrexate, and the combination of the two in the treatment of rheumatoid arthritis. A forty-eight-week controlled clinical trial with radiologic outcome assessment. Arthritis Rheum. 1995;38:1799–806.
Rau R, Schleusser B, Herborn G, Karger T. Long-term treatment of destructive rheumatoid arthritis with methotrexate. J Rheumatol. 1997;24:1881–9.
Weinblatt ME, Coblyn JS, Fox DA, Fraser PA, Holdsworth DE, Glass DN, et al. Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med. 1985;312:818–22.
Feagan BG, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart AH, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s Study Group Investigators. N Engl J Med. 2000;342:1627–32.
McDonald JWD, Tsoulis DJ, Macdonald JK, Feagan BG. Methotrexate for induction of remission in refractory Crohn’s disease. Cochrane Database Syst Rev. 2012;12:CD003459.
Marchetti M, Liberato NL. Biological therapies in Crohn’s disease: are they cost-effective? A critical appraisal of model-based analyses. Expert Rev Pharmacoecon Outcomes Res. 2014;14:815–24.
Loftus EV, Johnson SJ, Yu AP, Wu EQ, Chao J, Mulani PM. Cost-effectiveness of adalimumab for the maintenance of remission in patients with Crohn’s disease. Eur J Gastroenterol Hepatol. 2009;21:1302–9.
Holko P, Kawalec P, Pilc A. Cost-effectiveness analysis of Crohn’s disease treatment with vedolizumab and ustekinumab after failure of tumor necrosis factor-α antagonist. pharmacoeconomics [internet] . PharmacoEconomics. 2018;36:853–65. https://doi.org/10.1007/s40273-018-0653-2.
Bakhshai J, Bleu-Lainé R, Jung M, Lim J, Reyes C, Sun L, et al. The cost effectiveness and budget impact of natalizumab for formulary inclusion. J Med Econ. 2010;13:63–9.
Augustine JM, Lee JK, Armstrong EP. Health outcomes and cost-effectiveness of certolizumab pegol in the treatment of Crohn’s disease. Expert Rev Pharmacoecon Outcomes Res. 2014;14:599–609.
Aliyev ER, Hay JW, Hwang C. Cost-Effectiveness comparison of ustekinumab, infliximab, or adalimumab for the treatment of moderate-severe Crohn’s disease in biologic-Naïve patients. Pharmacotherapy. 2019;39:118–28.
Česká gastroenterologická společnost ČLS JEP [Internet]. https://www.cgs-cls.cz/. Accessed 27 Jun 2019.
Wilson MR, Azzabi Zouraq I, Chevrou-Severac H, Selby R, Kerrigan MC. Cost-effectiveness of vedolizumab compared with conventional therapy for ulcerative colitis patients in the UK. Clin Outcomes Res CEOR. 2017;9:641–52.
Wilson MR, Bergman A, Chevrou-Severac H, Selby R, Smyth M, Kerrigan MC. Cost-effectiveness of vedolizumab compared with infliximab, adalimumab, and golimumab in patients with ulcerative colitis in the United Kingdom. Eur J Health Econ HEPAC Health Econ Prev Care. 2018;19:229–40.
Overview | Vedolizumab for treating moderately to severely active Crohn’s disease after prior therapy | Guidance | NICE [Internet]. https://www.nice.org.uk/guidance/ta352. Accessed 28 Jun 2019
Tsai HH, Punekar YS, Morris J, Fortun P. A model of the long-term cost effectiveness of scheduled maintenance treatment with infliximab for moderate-to-severe ulcerative colitis. Aliment Pharmacol Ther. 2008;28:1230–9.
Holko P, Kawalec P, Pilc A. Cost-effectiveness analysis of Crohn’s disease treatment with vedolizumab and ustekinumab after failure of tumor necrosis factor-α antagonist. PharmacoEconomics. 2018;36:853–65.
Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, Chen DM, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4:621–30.
PE Guidelines Around The World: Czech Republic [Internet]. https://tools.ispor.org/PEguidelines/countrydet.asp?c=47&t=2. Accessed 28 Jun 2019.
Regulation of prices and reimbursements for pharmaceuticals, State Institute for Drug Control [Internet]. http://www.sukl.eu/medicines/regulation-of-prices-and-reimbursements-for-pharmaceuticals?highlightWords=sp-cau-028. Accessed 12 Jul 2019.
ISPOR-Good Practices for Outcomes Research [Internet]. http://www.ispor.org/heor-resources/good-practices-for-outcomes-research. Accessed 28 Jun 2019.
Consolidated Health Economic Evaluation Reporting Standards (CHEERS)-Explanation and Elaboration [Internet]. ISPOR Int. Soc. Pharmacoeconomics Outcomes Res. https://www.ispor.org/heor-resources/good-practices-for-outcomes-research/article/consolidated-health-economic-evaluation-reporting-standards-(cheers)-explanation-and-elaboration. Accessed 28 Jun 2019.
METOJECT, 50MG/ML INJ SOL ISP 1X0,30ML+J I, State Institute for Drug Control [Internet]. http://www.sukl.eu/modules/medication/detail.php?code=0128246&tab=prices. Accessed 23 Jul 2020.
Latimer NR. Survival analysis for economic evaluations alongside clinical trials—extrapolation with patient-level data [Internet]. London: National Institute for Health and Care Excellence (NICE); 2013. http://www.ncbi.nlm.nih.gov/books/NBK395885/. Accessed 13 Feb 2018.
Latimer NR. Survival analysis for economic evaluations alongside clinical trials–extrapolation with patient-level data: inconsistencies, limitations, and a practical guide. Med Decis Mak Int J Soc Med Decis Mak. 2013;33:743–54.
Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel J-F, Sands BE, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–21.
List of reimbursed medicinal products, State Institute for Drug Control [Internet]. http://www.sukl.cz/sukl/seznam-cen-a-uhrad-lp-pzlu-k-1-7-2019. Accessed 12 Jul 2019.
State Institute for Drug Control [Internet]. http://www.sukl.eu/modules/procedures/detail.php?spzn=sukls164619%2F2014&lang=2. Accessed 3 Jul 2019.
HUMIRA, 40MG INJ SOL 2X0,4ML, State Institute for Drug Control [Internet]. http://www.sukl.eu/modules/medication/detail.php?code=0209097&tab=prices&lang=2. Accessed 10 Aug 2020.
Central bank exchange rate fixing-Czech National Bank [Internet]. https://www.cnb.cz/en/financial-markets/foreign-exchange-market/central-bank-exchange-rate-fixing/central-bank-exchange-rate-fixing/. Accessed 28 Jun 2019.
Buxton MJ, Lacey LA, Feagan BG, Niecko T, Miller DW, Townsend RJ. Mapping from disease-specific measures to utility: an analysis of the relationships between the Inflammatory Bowel Disease Questionnaire and Crohn’s Disease Activity Index in Crohn’s disease and measures of utility. Value Health J Int Soc Pharmacoecon Outcomes Res. 2007;10:214–20.
Life tables [Internet]. Life Tables. https://www.czso.cz/csu/czso/life_tables. Accessed 28 Jun 2019.
The reference case | Guide to the methods of technology appraisal 2013 | Guidance | NICE [Internet]. NICE. https://www.nice.org.uk/process/pmg9/chapter/the-reference-case#exploring-uncertainty. Accessed 23 Jul 2020.
Overview | Ustekinumab for moderately to severely active Crohn’s disease after previous treatment | Guidance | NICE [Internet]. https://www.nice.org.uk/guidance/ta456. Accessed 3 Jul 2019.
Overview | Infliximab and adalimumab for the treatment of Crohn’s disease | Guidance | NICE [Internet]. https://www.nice.org.uk/guidance/ta187. Accessed 3 Jul 2019.
Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020;14:4–22.
Cesarini M, Festa S, Papi C. Methotrexate in Crohn’s disease: a new face for an old drug? Expert Rev Gastroenterol Hepatol. 2016;10:1135–44.
Acknowledgements
We thank Thomas O. Secrest for proofreading and Verka Horackova for graphical support. We also thank the reviewers for their insightful comments, which increased the quality of the manuscript. I thank my son Benjamin and loving wife Kveta for their unbelievable support (TM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Medac Czech Republic partly supported the preparation of the analysis for the purposes of obtaining reimbursement from public health insurance in the Czech Republic. However, the preparation of the publication was not financially supported.
Conflicts of interest
TM and BD are employees of Value Outcomes. Value Outcomes is a consultancy company working for the pharmaceutical industry in the field of market access, health economics, and outcomes research. TD is the director and owner of Value Outcomes.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The authors confirm that the data supporting the findings of the analysis are publicly available within the article, its supplementary materials and the articles referenced in the manuscript.
Code availability
The cost-effectiveness model is available from the author TM upon request.
Author contributions
All authors contributed to the analysis conception, its design, and the material preparation and data collection. The analysis was performed by TM, clinical background was provided by BD, and collection of inputs was done by all authors. The manuscript was written by BD and TM, all authors commented on each version of the manuscript. All authors read and approved the final manuscript. TD supervised the process.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mlcoch, T., Decker, B. & Dolezal, T. Cost-Effectiveness Analysis of Parenteral Methotrexate for the Treatment of Crohn’s Disease. Appl Health Econ Health Policy 19, 593–604 (2021). https://doi.org/10.1007/s40258-020-00628-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-020-00628-y