Abstract
Background and objective
The EORTC Quality of Life Utility Measure-Core 10 Dimensions (QLU-C10D) is a new multi-attribute utility instrument derived from the EORTC Quality of Life Questionnaire-Core 30 (QLQ-C30), a widely used cancer-specific quality-of-life questionnaire. It covers ten dimensions: physical, role functioning, social, emotional functioning, pain, fatigue, sleep, appetite, nausea and bowel problems. To allow national health preferences to be reflected, country-specific valuations are being performed through collaboration between the Multi-Attribute Utility Cancer (MAUCa) Consortium and the EORTC. The aim of this study was to determine the utility weights for health states in the French version of the QLU-C10D.
Methods
Valuations were run in a web-based setting in a general population sample of 1033 adults. Utilities were elicited using a discrete-choice experiment (DCE). Data were analyzed by conditional logistic regression and mixed logits.
Results
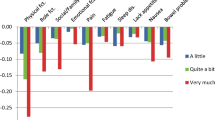
The sample was representative of the general French population in terms of gender and age. Dimensions with the largest impact on utility weights were, in this order: physical functioning, pain and emotional functioning. The impact on utilities was lower for role functioning, nausea, bowel problems and social functioning. The dimensions of sleep, fatigue and lacking appetite were associated with the smallest utility decrement.
Conclusion
The results of the present study provide utility weights for the QLU-C10D and offer interesting prospects, as some cancer-specific dimensions also received sizeable utility weights (nausea and bowel problems). In fact, the EQ-5D and the HUI 3 are recommended in France and commonly used for cancer-related CUA; however, both these instruments are generic. The availability of a new cancer-specific utility instrument, such as the QLU-C10D, could improve the quality and the pertinence of future CUA in oncology


Similar content being viewed by others
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Drummond MF, Mason AR. European perspective on the costs and cost-effectiveness of cancer therapies. J Clin Oncol. 2007;25:191–5. https://doi.org/10.1200/JCO.2006.07.8956.
Greenberg D, Earle C, Fang C-H, Eldar-Lissai A, Neumann PJ. When is cancer care cost-effective? A systematic overview of cost-utility analyses in oncology. J Natl Cancer Inst. 2010;102:82–8. https://doi.org/10.1093/jnci/djp472.
Frederix GWJ, Severens JL, Hövels AM, Raaijmakers JAM, Schellens JHM. The cloudy crystal ball of cost-effectiveness studies. Value Health. 2013;16:1100–2. https://doi.org/10.1016/j.jval.2013.06.012.
Guidelines for the economic evaluation of health technologies: Canada n.d. https://www.cadth.ca/. Accessed17 Sept 2019.
National Institute for Health and Care Excellence (NICE). National Institute for Health and Care Excellence (NICE) n.d. https://publications.nice.org.uk/pmg9.
Haute Autorité de Santé - Choices in methods for economic evaluation - A methodological guide n.d. https://www.has-sante.fr/portail/jcms/r_1499251/en/choices-in-methods-for-economic-evaluation. Accessed 17 Sept 2019.
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–36. https://doi.org/10.1007/s11136-011-9903-x.
Chevalier J, de Pouvourville G. Valuing EQ-5D using time trade-off in France. Eur J Health Econ. 2013;14:57–66. https://doi.org/10.1007/s10198-011-0351-x.
Feeny D, Furlong W, Boyle M, Torrance GW. Multi-attribute health status classification systems. Health Util Index Pharmacoecon. 1995;7:490–502.
Torrance GW, Furlong W, Feeny D, Boyle M. Multi-attribute preference functions. Health Util Index Pharmacoecon. 1995;7:503–20.
Torrance GW, Feeny DH, Furlong WJ, Barr RD, Zhang Y, Wang Q. Multiattribute utility function for a comprehensive health status classification system. Health Utilities Index Mark 2. Med Care. 1996;34:702–22.
Brazier J, Dixon S. The use of condition specific outcome measures in economic appraisal. Health Econ. 1995;4:255–64.
Stolk EA, Busschbach JJV. Validity and feasibility of the use of condition-specific outcome measures in economic evaluation. Qual Life Res. 2003;12:363–71.
Wiebe S, Guyatt G, Weaver B, Matijevic S, Sidwell C. Comparative responsiveness of generic and specific quality-of-life instruments. J Clin Epidemiol. 2003;56:52–60.
King MT, Costa DSJ, Aaronson NK, Brazier JE, Cella DF, Fayers PM, et al. QLU-C10D: a health state classification system for a multi-attribute utility measure based on the EORTC QLQ-C30. Qual Life Res. 2016;25:625–36. https://doi.org/10.1007/s11136-015-1217-y.
Norman R, Viney R, Aaronson NK, Brazier JE, Cella D, Costa DSJ, et al. Using a discrete choice experiment to value the QLU-C10D: feasibility and sensitivity to presentation format. Qual Life Res. 2016;25:637–49. https://doi.org/10.1007/s11136-015-1115-3.
King MT, Viney R, Simon Pickard A, Rowen D, Aaronson NK, Brazier JE, et al. Australian utility weights for the EORTC QLU-C10D, a multi-attribute utility instrument derived from the cancer-Specific quality of life questionnaire, EORTC QLQ-C30. Pharmacoeconomics. 2018;36:225–38. https://doi.org/10.1007/s40273-017-0582-5.
Norman R, Cronin P, Viney R, King M, Street D, Ratcliffe J. International comparisons in valuing EQ-5D health states: a review and analysis. Value Health. 2009;12:1194–200. https://doi.org/10.1111/j.1524-4733.2009.00581.x.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76.
Mulhern B, Norman R, De Abreu LR, Malley J, Street D, Viney R. Investigating the relative value of health and social care related quality of life using a discrete choice experiment. Soc Sci Med. 2019;233:28–37. https://doi.org/10.1016/j.socscimed.2019.05.032.
National Institute of Statistics and Economic Studies - France (INSEE) n.d. https://www.insee.fr/en/default.asp. Accessed 17 Sept 2019.
Ware JE, Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol. 1998;51:903–12.
Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SLT, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. 2002;32:959–76.
Colbourn CJ, Dinitz JH. Handbook of combinatorial designs, second edition (Discrete mathematics and its applications). London: Chapman & Hall/CRC; 2006.
Street DJ, Burgess L. The construction of optimal stated choice experiments: theory and methods. Hoboken: Wiley; 2007.
Viney R, Norman R, Brazier J, Cronin P, King MT, Ratcliffe J, et al. An Australian discrete choice experiment to value eq-5d health states. Health Econ. 2014;23:729–42. https://doi.org/10.1002/hec.2953.
Bansback N, Brazier J, Tsuchiya A, Anis A. Using a discrete choice experiment to estimate health state utility values. J Health Econ. 2012;31:306–18. https://doi.org/10.1016/j.jhealeco.2011.11.004.
Nay O, Béjean S, Benamouzig D, Bergeron H, Castel P, Ventelou B. Achieving universal health coverage in France: policy reforms and the challenge of inequalities. Lancet. 2016;387:2236–49. https://doi.org/10.1016/S0140-6736(16)00580-8.
Haute Autorité de Santé (French National Authority for Health) n.d. https://www.has-sante.fr/portail/jcms/r_1455081/Home-page. Accessed 20 Aug 2019.
Norman R, Mercieca-Bebber R, Rowen D, Brazier JE, Cella D, Pickard AS, et al. UK utility weights for the EORTC QLU-C10D. Health Econ. 2019. https://doi.org/10.1002/hec.3950.
Kemmler G, Gamper E, Nerich V, Norman R, Viney R, Holzner B, et al. German value sets for the EORTC QLU-C10D, a cancer-specific utility instrument based on the EORTC QLQ-C30. Qual Life Res. 2019. https://doi.org/10.1007/s11136-019-02283-w.
Ratcliffe J, Wang K, Barr C, Norman R, George S, Whitehead C. Using eye tracking technology to investigate the impact of mild cognitive impairment on preferences for EQ-5D health states: an exploratory study with older people in memory clinics. Appl Health Econ Health Policy n.d. (in press)
Whitty JA, Walker R, Golenko X, Ratcliffe J. A think aloud study comparing the validity and acceptability of discrete choice and best worst scaling methods. PLoS ONE. 2014;9:e90635. https://doi.org/10.1371/journal.pone.0090635.
Mulhern B, Norman R, Street DJ, Viney R. One method, many methodological choices: a structured review of discrete-choice experiments for health state valuation. Pharmacoeconomics. 2019;37:29–43. https://doi.org/10.1007/s40273-018-0714-6.
Craig BM, Rand K, Bailey H, Stalmeier PFM. Quality-adjusted life-years without constant proportionality. Value Health. 2018;21:1124–31. https://doi.org/10.1016/j.jval.2018.02.004.
Jonker MF, Donkers B, de Bekker-Grob EW, Stolk EA. Advocating a paradigm shift in health-state valuations: the estimation of time-preference corrected QALY tariffs. Value Health. 2018;21:993–1001. https://doi.org/10.1016/j.jval.2018.01.016.
Costet N, Le Galès C, Buron C, Kinkor F, Mesbah M, Chwalow J, et al. French cross-cultural adaptation of the Health Utilities Indexes Mark 2 (HUI2) and 3 (HUI3) classification systems. Clinical and Economic Working Groups. Qual Life Res. 1998;7:245–56.
Nerich V, Saing S, Gamper EM, Kemmler G, Daval F, Pivot X, et al. Cost-utility analyses of drug therapies in breast cancer: a systematic review. Breast Cancer Res Treat. 2016;159:407–24. https://doi.org/10.1007/s10549-016-3924-7.
Nerich V, Saing S, Gamper E-M, Holzner B, Pivot X, Viney R, et al. Critical appraisal of health-state utility values used in breast cancer-related cost-utility analyses. Breast Cancer Res Treat. 2017;164:527–36. https://doi.org/10.1007/s10549-017-4283-8.
Gérard C, Fagnoni P, Vienot A, Borg C, Limat S, Daval F, et al. A systematic review of economic evaluation in pancreatic ductal adenocarcinoma. Eur J Cancer. 2017;86:207–16. https://doi.org/10.1016/j.ejca.2017.08.035.
Toumi M, Jarosławski S, Chouhaid C, Fallissard B, Auquier P. Patient-reported outcomes in oncology, beyond randomized controlled trials. Recent Results Cancer Res. 2019;213:57–655. https://doi.org/10.1007/978-3-030-01207-6_5.
Mouillet G, Fritzsch J, Paget-Bailly S, Pozet A, Es-Saad I, Meurisse A, et al. Health-related quality of life assessment for patients with advanced or metastatic renal cell carcinoma treated with a tyrosine kinase inhibitor using electronic patient-reported outcomes in daily clinical practice (QUANARIE trial): study protocol. Health Qual Life Outcomes. 2019;17:25. https://doi.org/10.1186/s12955-019-1085-1.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Prof. VN and GK. The first draft of the manuscript was written by Prof. VN and Dr GK and all co-authors commented on previous versions of the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Funding
This research project was funded by Grant Number 002-2014 of the EORTC Quality of Life Group. Professor King was supported by the Australian Government through Cancer Australia.
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Medical University of Innsbruck Ethics Committee, number 20151207–1336.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nerich, V., Gamper, E.M., Norman, R. et al. French Value-Set of the QLU-C10D, a Cancer-Specific Utility Measure Derived from the QLQ-C30. Appl Health Econ Health Policy 19, 191–202 (2021). https://doi.org/10.1007/s40258-020-00598-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-020-00598-1