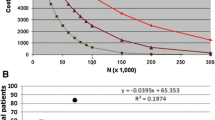
Abstract
Background
When pharmaceuticals are administered based on patient characteristics (for example weight or body surface area), an amount of product will be unused and must be disposed of. This wastage represents inefficiency and can distort decision making.
Methods
We present a method for the analysis of optimum fill volumes of pharmaceuticals to minimise wastage across a patient population, using publicly available data. Wastage for patients at each ‘step’ e.g. by kg of bodyweight is calculated, the frequency of each of these steps in the structure of the population is then estimated using the method of moments, with wastage then estimated for each ‘step’ multiplied by its prevalence. Illustrative examples of pembrolizumab and cabazitaxel show how wastage could be reduced using UK population data, whilst simultaneously reducing administrative burden.
Results
Changing the available vial sizes for pembrolizumab (available as 50 mg/100 mg vials) to 70 mg/100 mg, wastage could be cut from 13.3% to 8.7%. For cabazitaxel (only 60 mg vials available), increasing the fill to 70 mg could reduce wastage from 19.4% to 18.8%, or alternatively, adding a 12.5 mg vial reduce this to 6.5%. A secondary finding is that wastage is higher when the larger vial size is perfectly divisible by the smaller vial size.
Conclusions
Reductions in wastage have the potential to reduce the cost of manufacturing medicines, which is not necessarily low for novel products. These cost reductions could lead to increased profit (at the same prices), constant profit with a better return rate (at lower prices), or a combination of the two. Most importantly, they would improve the efficiency of the health-care sector, increasing funding available to treat patients.

Similar content being viewed by others
References
Fojo T, Grady C. How much is life worth: cetuximab, non-small cell lung cancer, and the $440 billion question. JNCI J Natl Cancer Inst. 2009;101:1044–8. https://doi.org/10.1093/jnci/djp177.
Prasad V, Mailankody S. Research and development spending to bring a single cancer drug to market and revenues after approval. JAMA Intern Med. https://doi.org/10.1001/jamainternmed.2017.3601.
Paul SM, Mytelka DS, Dunwiddie CT, et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov. 2010;9:203. https://doi.org/10.1038/nrd3078.
Eichler H-G, Oye K, Baird LG, et al. Adaptive licensing: taking the next step in the evolution of drug approval. Clin Pharmacol Ther. 2012;91:426–37. https://doi.org/10.1038/clpt.2011.345.
Bach PB, Conti RM, Muller RJ, et al. Overspending driven by oversized single dose vials of cancer drugs. BMJ. 2016;352:i788. https://doi.org/10.1136/bmj.i788.
Hatswell AJ, Porter J, Lee D, et al. The cost of costing treatments incorrectly: errors in the application of drug prices in economic evaluation due to failing to account for the distribution of patient weight. Value Health. 2016;19:1055–8. https://doi.org/10.1016/j.jval.2016.04.013.
Truong J, Cheung MC, Mai H, et al. The impact of cancer drug wastage on economic evaluations. Cancer. 2017;123:3583–90. https://doi.org/10.1002/cncr.30807.
Goldstein DA, Gordon N, Davidescu M, et al. A Phamacoeconomic analysis of personalized dosing vs fixed dosing of pembrolizumab in firstline PD-L1-positive non-small cell lung cancer. J Natl Cancer Inst 2017. https://doi.org/10.1093/jnci/djx063.
Gilbar PJ, Chambers CR, Gilbar EC. Opportunities to significantly reduce expenditure associated with cancer drugs. Future Oncol. 2017;13:1311–22. https://doi.org/10.2217/fon-2017-0033.
Williamson S, Polwart C. Guidelines for the dose banding of cancer chemotherapy. 2011.http://www.necn.nhs.uk/wp-content/uploads/2012/11/NECN-dose-banding-guidelines-version-1_5.pdf. Accessed 13 Dec 2017.
European Medicines Agency. Pembrolizumab summary of product characteristics. https://www.ema.europa.eu/documents/product-information/keytruda-epar-product-information_en.pdf. Accessed 2 Oct 2018.
European Medicines Agency. Cabazitaxel summary of product characteristics. https://www.ema.europa.eu/documents/product-information/jevtana-epar-product-information_en.pdf. Accessed 2 Oct 2018.
Fuller E, Mindell J, Prior G (eds). Health survey for England 2015. London: NHS Digital; 2016. https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/health-survey-for-england-2015. Accessed 10 May 2018.
National Institute for Health and Care Excellence. Cabazitaxel for hormone-relapsed metastatic prostate cancer treated with docetaxel: Final Appraisal Determination. 2016. https://www.nice.org.uk/guidance/ta391/resources/cabazitaxel-for-hormonerelapsed-metastatic-prostate-cancer-treated-with-docetaxel-pdf-82602905134021. Accessed 13 Dec 2017.
Food and Drug Administration. Allowable excess volume and labeled vial fill size in injectable drug and biological products guidance for industry. 8.
Doble B, Payne R, Harshfield A, et al. prescription lengths in primary care for common, chronic conditions in the UK. Open Access 9.
Author information
Authors and Affiliations
Contributions
The analysis was performed by AJH and JKP. The manuscript with drafted by AJH and JKP with the final version approved by AJH and JKP.
Corresponding author
Ethics declarations
For transparency AJH has worked with the majority of large pharmaceutical companies and JP is currently an employee of Amgen Inc. However, this work was performed prior to his employment and is not sponsored, endorsed, or funded by Amgen Inc. in any form—for this reason JP had no affiliation at the time of this work. No funding was received for the research contained in this manuscript.
Funding
None.
Data sharing
All data used in the analysis are publicly available.
Rights and permissions
About this article
Cite this article
Hatswell, A.J., Porter, J.K. Reducing Drug Wastage in Pharmaceuticals Dosed by Weight or Body Surface Areas by Optimising Vial Sizes. Appl Health Econ Health Policy 17, 391–397 (2019). https://doi.org/10.1007/s40258-018-0444-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-018-0444-0