Abstract
Background
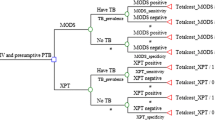
The Xpert® MTB/RIF (Xpert) test has been shown to be effective and cost-effective for diagnosing tuberculosis (TB) under conditions with high HIV prevalence and HIV-TB co-infection but less is known about Xpert’s cost in low HIV prevalence settings. Cambodia, a country with low HIV prevalence (0.7%), high TB burden, and low multidrug-resistant (MDR) TB burden (1.4% of new TB cases, 11% of retreatment cases) introduced Xpert into its TB diagnostic algorithms for people living with HIV (PLHIV) and people with presumptive MDR TB in 2012. The study objective was to estimate these algorithms’ costs pre- and post-Xpert introduction in four provinces of Cambodia.
Methods
Using a retrospective, ingredients-based microcosting approach, primary cost data on personnel, equipment, maintenance, supplies, and specimen transport were collected at four sites through observation, records review, and key informant consultations.
Results
Across the sample facilities, the cost per Xpert test was US$33.88–US$37.11, clinical exam cost US$1.22–US$1.84, chest X-ray cost US$2.02–US$2.14, fluorescent microscopy (FM) smear cost US$1.56–US$1.93, Ziehl–Neelsen (ZN) smear cost US$1.26, liquid culture test cost US$11.63–US$22.83, follow-on work-up for positive culture results and Mycobacterium tuberculosis complex (MTB) identification cost US$11.50–US$14.72, and drug susceptibility testing (DST) cost US$44.26. Specimen transport added US$1.39–US$5.21 per sample. Assuming clinician adherence to the algorithms and perfect test accuracy, the normative cost per patient correctly diagnosed under the post-Xpert algorithms would be US$25–US$29 more per PLHIV and US$34–US$37 more per person with presumptive MDR TB (US$41 more per PLHIV when accounting for variable test sensitivity and specificity).
Conclusions
Xpert test unit costs could be reduced through lower cartridge prices, longer usable life of GeneXpert® (Cepheid, USA) instruments, and increased test volumes; however, epidemiological and test eligibility conditions in Cambodia limit the number of specimens received at laboratories, leading to sub-optimal utilization of current instruments. Improvements to patient referral and specimen transport could increase test volumes and reduce Xpert test unit costs in this setting.
Similar content being viewed by others
References
World Health Organization. Global Tuberculosis Report 2014. Geneva: World Health Organization; 2014.
World Health Organization. Rapid implementation of the Xpert MTB/RIF diagnostic test: Technical and operational ‘How to’ practical considerations. Geneva: World Health Organization; 2011.
World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system: policy statement. Geneva: World Health Organization; 2011.
Padmapriyadarsini C, Narendran G, Swaminathan S. Diagnosis and treatment of tuberculosis in HIV co-infected patients. Indian J Med Research. 2011;134(6):16.
World Health Organization. Tuberculosis diagnostics Xpert MTB/RIF test Fact Sheet. Geneva: World Health Organization; 2013.
Jones BE, et al. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis. 1993;148(5):6.
Perlman DC, et al. Variation of chest radiographic patterns in pulmonary tuberculosis by degree of human immunodeficiency virus-related immunosuppression. Clin Infect Dis. 1997;25(2):5.
Whalen C, et al. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med. 1995;151(1):7.
Gandhi NR, et al. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med. 2010;181(1):7.
Piatek A, et al. GeneXpert for TB diagnosis: planned and purposeful implementation. Glob Health Sci Pract. 2013;1(1):6.
World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children. Policy update. Geneva: World Health Organization; 2013.
Boehme CC, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert® MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377(9776):11.
Boehme CC, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363(11):11.
Lawn SD, Nicol MP. Xpert® MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiology. 2011;6(9):16.
Carriquiry G, et al. A diagnostic accuracy study of Xpert® (R) MTB/RIF in HIV-positive patients with high clinical suspicion of pulmonary tuberculosis in lima. Peru. PLoS ONE. 2012;7:9.
Chang K, et al. Rapid and effective diagnosis of tuberculosis and rifampicin resistance with Xpert® MTB/RIF assay: a meta-analysis. J Infect. 2012;64(6):9.
Yoon C, et al. Impact of Xpert® MTB/RIF testing on tuberculosis management and outcomes in hospitalized patients in Uganda. PLoS ONE. 2012;7:11.
Vassall A, et al. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high burden countries: a cost-effectiveness analysis. PLoS Med. 2011;8(11):e1001120.
Andrews JR, et al. The cost-effectiveness of routine tuberculosis screening with Xpert MTB/RIF prior to initiation of antiretroviral therapy: a model-based analysis. AIDS. 2012;26(8):987–95.
Menzies N, et al. Population health impact and cost-effectiveness of tuberculosis diagnosis with Xpert MTB/RIF: a dynamic simulation and economic evaluation. PLoS Med. 2012;9(11):e1001347.
Choi HW, et al. Cost-effectiveness of Xpert® MTB/RIF for diagnosing pulmonary tuberculosis in the United States. Int J Tuberc Lung Dis. 2013;17(10):1328–35.
Pantoja A, et al. Xpert MTB/RIF for diagnosis of tuberculosis and drug-resistant tuberculosis: a cost and affordability analysis. Eur Respir J. 2012;42(3):708–20.
Abimbola TO, et al. Cost-effectiveness of tuberculosis diagnostic strategies to reduce early mortality among persons with advanced HIV infection initiating antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;60(1):e1–7.
UNAIDS. The Gap Report. Geneva: Joint United Nations Programme on HIV/AIDS; 2014.
Auld SC, et al. Rollout of Xpert® MTB/RIF in northwest Cambodia for the diagnosis of tuberculosis among PLHA. Public Health Action. 2014;4(4):216–21.
US Bureau of Economic Analysis. Gross Domestic Product Implicit Price Deflator [GDPDEF]. 2018FRED. Louis: Federal Reserve Bank of St; 2018.
Steingart KR, et al. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6:570–81.
Smit P, et al. Systematic review of the use of dried blood spots for monitoring HIV viral load and for early infant diagnosis. PLoS ONE. 2014;9(3):e86461.
Van Rie A, et al. Point-of-care Xpert® MTB/RIF for smear-negative tuberculosis suspects at a primary care clinic in South Africa. Int J Tuberc Lung Dis. 2013;17(3):368–72.
Schnippel K, et al. Scaling up Xpert MTB/RIF technology: the costs of laboratory—vs. clinic-based roll-out in South Africa. Trop Med Int Health. 2012;17:1142–51.
Atif M, et al. Determination of chest X-ray cost using activity based costing approach at Penang General Hospital, Malaysia. Pan Afr Med J. 2012;12:40.
Pang Y, et al. Cost-effectiveness comparison of genechip and conventional drug susceptibility test for detecting multidrug-resistant tuberculosis in China. PLoS ONE. 2013;8:7.
Acknowledgements
The authors would like to acknowledge the input and feedback received from staff at the Cambodia National TB Program (CENAT), Dr. Kanara Nong and Mr. Huot Uong of CDC/Cambodia, and respondents at cost data collection sites in Battambang Provincial Referral Hospital, Mongkol Borei Provincial Referral Hospital, Mong Russey Operational District Hospital, and the CENAT National Laboratory.
Data Availability Statement
The data that support the findings of this study are not publicly available due to them containing information considered procurement sensitive by the Cambodia National TB Program (CENAT). The data are, however, available on reasonable request from the corresponding author (SWP) and with permission of CENAT.
Author information
Authors and Affiliations
Contributions
Conceptualization and design of the study: SWP, MC, WPK, DW, BM. Acquisition of data: SWP, CH, BM. Analysis of data: SWP, MC. Interpretation of data and analysis: SWP, MC, CH, WPK, DW, BM. Drafting of the manuscript: SWP. Revisions of the manuscript for important intellectual content: SWP, MC, CH, WPK, DW, BM. Final approval of the manuscript for publication: SWP, MC, CH, WPK, DW, BM.
Corresponding author
Ethics declarations
Funding
This work was supported by funding provided by the US Agency for International Development and the US Centers for Disease Control and Prevention, in addition to funding from the President’s Emergency Plan for AIDS Relief (PEPFAR) through the US Centers for Disease Control and Prevention. The findings and conclusions in this presentation are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Ethical review
The US Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA, and the Cambodia National Tuberculosis Control Program (CENAT) determined the study to be a program evaluation and not research involving human subjects, and therefore no IRB approval was required.
Conflicts of interest
Sarah Wood Pallas, Marissa Courey, Chhaily Hy, William Perry Killam, Dora Warren, and Brittany Moore declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pallas, S.W., Courey, M., Hy, C. et al. Cost Analysis of Tuberculosis Diagnosis in Cambodia with and without Xpert® MTB/RIF for People Living with HIV/AIDS and People with Presumptive Multidrug-resistant Tuberculosis. Appl Health Econ Health Policy 16, 537–548 (2018). https://doi.org/10.1007/s40258-018-0397-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-018-0397-3