Abstract
Background and Objectives
Budget impact analysis (BIA) has become an essential part of economic evaluation within health technology assessment. Several disease-modifying therapies (DMTs) are now available for the treatment of multiple sclerosis (MS). This study sought to identify the inputs and assumptions used in existing BIAs for DMTs in the UK, and the uncertainty and variation in these, to allow critique within the context of UK policy.
Methods
MEDLINE and the Economic Evaluations Database from the Cochrane Library were searched systematically on 15 December 2014 to identify BIAs of DMTs licensed for MS in the UK. In addition, the National Institute for Health and Care Excellence (NICE) and National Health Service (NHS) England websites were searched for relevant publications and grey literature searching was undertaken. Sources and assumptions from the included analyses were extracted, compared and critiqued.
Results
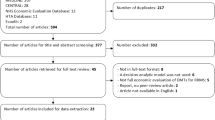
The database searches produced 115 de-duplicated results. An additional 12 results were identified from the NICE and NHS England websites. No BIAs of DMTs for MS in the UK were identified in the literature. All ten included studies were from the NICE website, comprising manufacturer submissions for each DMT and corresponding NICE costing templates. There are considerable uncertainties in the inputs and assumptions used in the BIAs, but limited sensitivity analyses were undertaken.
Conclusions
Data limitations were not highlighted in the results, failing to present the uncertainty in the results to users clearly. It is to be welcomed that NICE has recently consulted on a process change to allow additional critique of the costing templates.

Similar content being viewed by others
References
Sullivan SD, Mauskopf JA, Augustovski F, Caro J, Lee KM, Minchin M, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17(1):5–14.
van de Vooren K, Duranti S, Curto A, Garattini L. A critical systematic review of budget impact analyses on drugs in the EU countries. Appl Health Econ Health Policy. 2014;12(1):33–40.
Trueman P, Drummond M, Hutton J. Developing guidance for budget impact analysis. Pharmacoeconomics. 2001;19(6):609–21.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013.http://www.nice.org.uk/article/PMG9/chapter/Foreword. Accessed 30 Jan 2015.
National Institute for Health and Care Excellence. Assessing cost impact: methods guide. 2011. http://www.nice.org.uk/Media/Default/About/what-we-do/Into-practice/Costing_Manual_update_050811.pdf. Accessed 30 Jan 2015.
National Institute for Health and Care Excellence. Resource impact assessment methods guide consultation. 2015. https://www.nice.org.uk/Media/Default/About/what-we-do/Into-practice/costing/ria-method-guide-revision-V12-consultation.pdf. Accessed 11 Sep 2015.
NHS England. NHS five year forward view. 2014. https://www.england.nhs.uk/wp-content/uploads/2014/10/5yfv-web.pdf. Accessed 14 Apr 2016.
Nylander A, Hafler DA. Multiple sclerosis. J Clin Invest. 2012;122(4):1180–8.
Mackenzie IS, Morant SV, Bloomfield GA, MacDonald TM, O’Riordan J. Incidence and prevalence of multiple sclerosis in the UK 1990–2010: a descriptive study in the General Practice Research Database. J Neurol Neurosurg Psychiatry. 2014;85(1):76–84.
Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359(9313):1221–31.
Derwenskus J, Lublin FD. Future treatment approaches to multiple sclerosis. Handb Clin Neurol. 2014;122:563–77.
electronic Medicines Compendium (eMC). TYSABRI 300 mg concentrate for solution for infusion. 25 Nov 2013. http://www.medicines.org.uk/emc/medicine/18447. Accessed 30 Jan 2015.
electronic Medicines Compendium (eMC). Gilenya 0.5 mg hard capsules. 08 Sep 2014. http://www.medicines.org.uk/emc/medicine/24443/SPC/. Accessed 30 Jan 2015.
electronic Medicines Compendium (eMC). Tecfidera 240 mg gastro-resistant hard capsules. 27 Aug 2014. http://www.medicines.org.uk/emc/medicine/28593. Accessed 30 Jan 2015.
electronic Medicines Compendium (eMC). AUBAGIO 14 mg film-coated tablets. 22 Sep 2014. http://www.medicines.org.uk/emc/medicine/28533. Accessed 30 Jan 2015.
electronic Medicines Compendium (eMC). LEMTRADA 12 mg concentrate for solution for infusion. 15 Dec 2014. http://www.medicines.org.uk/emc/medicine/28917. Accessed 30 Jan 2015.
National Institute for Health and Care Excellence. Natalizumab (Tysabri®) for the treatment of adults with highly active relapsing remitting multiple sclerosis (TA127). London: NICE; 2007. https://www.nice.org.uk/guidance/ta127. Accessed 14 Apr 2016.
National Institute for Health and Care Excellence. Fingolimod for the treatment of highly active relapsing–remitting multiple sclerosis (TA254). London: NICE; 2012. https://www.nice.org.uk/guidance/ta254. Accessed 14 Apr 2016.
National Institute for Health and Care Excellence. Teriflunomide for treating relapsing–remitting multiple sclerosis (TA303). London: NICE; 2014. https://www.nice.org.uk/guidance/ta303. Accessed 14 Apr 2016.
National Institute for Health and Care Excellence. Dimethyl fumarate for treating relapsing–remitting multiple sclerosis (TA320). London: NICE; 2014. https://www.nice.org.uk/guidance/ta320. Accessed 14 Apr 2016.
National Institute for Health and Care Excellence. Alemtuzumab for treating relapsing–remitting multiple sclerosis (TA312). London: NICE; 2014. https://www.nice.org.uk/guidance/ta312. Accessed 14 Apr 2016.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9, W64.
Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Updated March 2011. http://handbook.cochrane.org/. Accessed 2 Mar 2015.
Sassi F, Archard L, McDaid D. Searching literature databases for health care economic evaluations: how systematic can we afford to be? Med Care. 2002;40(5):387–94.
Biogen Idec Ltd. Natalizumab (Tysabri®) for the treatment of adults with highly active relapsing remitting multiple sclerosis. Manufacturer submission of evidence to NICE. TA127. London: National Institute for Health and Clinical Excellence; 2007.
Biogen Idec Ltd. Dimethyl fumarate for the treatment of adult patients with relapsing remitting multiple sclerosis. Manufacturer submission of evidence. TA320. London: National Institute for Health and Care Excellence; 2013.
Genzyme. Alemtuzumab for the treatment of relapsing remitting multiple sclerosis in adults. Manufacturer submission of evidence. TA312. London: National Institute for Health and Care Excellence; 2013.
Genzyme. Teriflunomide for the treatment of relapsing-remitting multiple sclerosis in adults. Manufacturer submission of evidence. TA303. London: National Institute for Health and Care Excellence; 2013.
Novartis Pharmaceuticals UK Ltd. Fingolimod for the treatment of relapsing-remitting multiple sclerosis in adults. Manufacturer submission of evidence. TA254. London: National Institute for Health and Clinical Excellence; 2011.
Koutsouraki E, Costa V, Baloyannis S. Epidemiology of multiple sclerosis in Europe: a review. Int Rev Psychiatry. 2010;22(1):2–13.
Milo R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev. 2010;9(5):A387–94.
Multiple Sclerosis Trust. Multiple sclerosis information for health and social care professionals. 4th ed. Letchworth Garden City: MS Trust; 2011. http://www.mstrust.org.uk/health-professionals/practice-resources/ms-information-health-and-social-care-professionals. Accessed 14 Apr 2016.
Kobelt G, Berg J, Lindgren P, Kerrigan J, Russell N, Nixon R. Costs and quality of life of multiple sclerosis in the United Kingdom. Eur J Health Econ. 2006;7(Suppl 2):S96–104.
Zajicek JP, Ingram WM, Vickery J, Creanor S, Wright DE, Hobart JC. Patient-orientated longitudinal study of multiple sclerosis in south west England (The South West Impact of Multiple Sclerosis Project, SWIMS) 1: protocol and baseline characteristics of cohort. BMC Neurol. 2010;10:88.
Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, AFFIRM Investigators, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910.
Department of Health. Cost effective provision of disease modifying therapies for people with multiple sclerosis. London: Department of Health; 2002. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4012214.pdf. Accessed 14 Apr 2016.
Dee A, Hutchinson M, De La Harpe D. A budget impact analysis of natalizumab use in Ireland. Ir J Med Sci. 2012;181(2):199–204.
Health and Social Care Information Centre. Use of NICE appraised medicines in the NHS in England—experimental statistics. 2009. http://www.hscic.gov.uk/catalogue/PUB01400/use-nice-app-med-nhs-exp-stat-eng-exp.pdf. Accessed 14 Sep 2015.
ICJME. Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals. 2014. http://www.icmje.org/recommendations/browse/manuscript-preparation/preparing-for-submission.html#g. Accessed 11 Nov 2015.
Health and Social Care Information Centre. Use of NICE appraised medicines in the NHS in England—2012, experimental statistics. 2014. http://www.hscic.gov.uk/catalogue/PUB13413/use-nice-app-med-nhs-exp-stat-eng-12-rep.pdf. Accessed 14 Sep 2015.
Health and Social Care Information Centre. NICE technology appraisals in the NHS in England (innovation scorecard): to September 2014, experimental statistics. Estimates of predicted use compared to observed use. 2015. http://www.hscic.gov.uk/catalogue/PUB17559/nice-tech-apps-eng-may15-exp-inno-scor-est.pdf. Accessed 14 Sep 2015.
NHS England Clinical Reference Group for Neurosciences. Clinical commissioning policy: disease modifying therapies for patients with multiple sclerosis (MS). Reference: NHS ENGLAND/D04/P/b. Updated May 2014. https://www.england.nhs.uk/wp-content/uploads/2013/10/d04-p-b.pdf. Accessed 14 Apr 2016.
Author contributions
All authors were involved in the conception and design of all or some component(s) of the research. Acquisition of data was carried out by SM and JK. All authors participated at all or some step(s) of the review, analysis and interpretation of the outcomes. NA was responsible for development of the manuscript. All authors reviewed and commented on the draft manuscript, and reviewed its intellectual content. All authors approved the final version of the manuscript. NA will act as the overall guarantor.
The authors take full responsibility for the content of the paper. No assistance in the preparation of this article is to be declared.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
NA and FA are paid employees of Novartis Pharmaceuticals UK Ltd, Camberley, UK. SM and JK are paid employees of Costello Medical Consulting Ltd, Cambridge, UK, which was contracted by Novartis to undertake some of the work. This study was funded by Novartis Pharmaceuticals UK Ltd, Camberley, UK. The views presented in this paper are those of the authors, and do not necessarily reflect those of the study funders.
Rights and permissions
About this article
Cite this article
Montgomery, S., Kusel, J., Allen, F. et al. Paucity and Inconsistency: A Systematic Review and Critique of Budget Impact Analyses of Disease-Modifying Therapies for Multiple Sclerosis in the UK and the Implications for Policy in the UK. Appl Health Econ Health Policy 14, 545–558 (2016). https://doi.org/10.1007/s40258-016-0244-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-016-0244-3