Abstract
Background
Follicular lymphoma (FL) is the second most common type of lymphoid cancer in Western Europe.
Objective
The aim of this study was to evaluate the cost utility of rituximab–bendamustine treatment compared with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) treatment as a first-line therapy for patients with advanced FL in Spain.
Methods
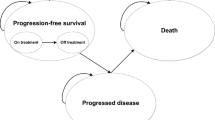
A Markov model was developed to estimate the cost effectiveness of rituximab–bendamustine compared with R-CHOP as first-line treatment for patients with advanced FL in the Spanish National Health System (NHS). Transitions between health states (progression-free, including induction and maintenance; first relapse; second relapse; and death) were allowed for the patient cohort in 4-week-long cycles. Clinical data for the extrapolation of progression-free survival curves were obtained from randomized trials. Mortality rates and utilities were obtained from the literature. Outcomes were measured as quality-adjusted life-years (QALYs). The total costs (€, 2013) included drug costs (ex-factory prices with mandatory deductions), disease management costs and adverse event-associated costs. Costs and outcomes were discounted at a 3 % annual rate. Probabilistic sensitivity analysis was performed using 10,000 Monte Carlo simulations to assess the model robustness.
Results
Treatment and administration costs during the induction phase were higher for rituximab–bendamustine (€17,671) than for R-CHOP (€11,850). At the end of the 25-year period, the rituximab–bendamustine first-line strategy had a total cost of €68,357 compared with €69,528 for R-CHOP. Health benefits were higher for rituximab–bendamustine treatment (10.31 QALYs) than for R-CHOP treatment (9.82 QALYs). In the probabilistic analysis, rituximab–bendamustine was the dominant strategy over treatment with R-CHOP in 53.4 % of the simulations.
Conclusion
First-line therapy with rituximab–bendamustine in FL patients was the dominant strategy over treatment with R-CHOP; it showed cost savings and higher health benefits for the Spanish NHS.




Similar content being viewed by others
References
Salar A, Fernández de Sevilla A, Romagosa V, Domingo-Claros A, Gonzalez-Barca E, De Sanjosé S, Pera J, Servitje O, Grañena A. Distribution and incidence rates of lymphoid neoplasms according to the REAL classification in a single institution. A prospective study of 940 cases. Eur J Haematol. 1997;59:231–7.
Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin’s lymphomas: distribution of the major subtypes differ by geographic locations. Non-Hodgkin’s Lymphoma Classification Project. Ann Oncol. 1998;9:717–20.
Zelenetz AD, Gordon LI, Wierda WG, Abramson JS, Advani RH, Andreadis CB, Bartlett N, Bellam N, Byrd JC, Czuczman MS, Fayad LE, Fisher RI, Glenn MJ, Harris NL, Hoppe RT, Horwitz SM, Kelsey CR, Kim YH, Krivacic S, LaCasce AS, Nademanee A, Porcu P, Press O, Rabinovitch R, Reddy N, Reid E, Sokol L, Swinnen LJ, Tsien C, Vose JM, Yahalom J, Zafar N, Dwyer M, Sundar H. Non-Hodgkin’s Lymphomas, Version 2.2014. J Natl Compr Canc Netw. 2014;12(6):916–46.
Dreyling M, Ghielmini M, Marcus R, Salles G, Vitolo U, ESMO Guidelines Working Group. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(6):vi59–63.
Ghielmini M, Vitolo U, Kimby E, Montoto S, Walewski J, Pfreundschuh M, Federico M, Hoskin P, McNamara C, Caligaris-Cappio F, Stilgenbauer S, Marcus R, Trneny M, Dreger P, Montserrat E,. Dreyling M, Panel Members of the 1st ESMO Consensus Conference on Malignant Lymphoma. ESMO Guidelines consensus conference on malignant lymphoma 2011 part 1: diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) and chronic lymphocytic leukemia (CLL) Ann Oncol. 2013;24:561–76.
López-Guillermo A, Caballero D, Canales M, Provencio M, Rueda A, Salar A. Spanish Hematology and Hemotherapy Association; Oncological Group for the Treatment of Lymphatic Diseases; Spanish Lymphomas/Autologous Bone Marrow Transplant Group. Clinical practice guidelines for first-line/after-relapse treatment of patients with follicular lymphoma. Leuk Lymphoma. 2011;52(Suppl 3):1–14.
Caballero D, Canales M, Provencio M, Rueda A, Salar A, López-Guillermo A. Guía de Práctica Clínica para el tratamiento del Linfoma Folicular. Available at URL: http://www.guiasalud.es/GPC/GPC_474_Linfoma_folicular.pdf. Accessed on 2 June 2014.
Witzens-Harig M, Foá R, Di Rocco A, van Hazel G, Chamone DF, Rowe JM, Arcaini L, Poddubnaya I, Ho AD, Ivanova V, Vranovsky A, Thurley D, Oertel S. Maintenance with rituximab is safe and not associated with severe or uncommon infections in patients with follicular lymphoma: results from the phase IIIb MAXIMA study. Ann Hematol. 2014;93(10):1717–24. doi:10.1007/s00277-014-2103-3.
Nastoupil LJ, Sinha R, Byrtek M, Zhou X, Taylor MD, Friedberg JW, Link BK, Cerhan JR, Dawson K, Flowers CR. The use and effectiveness of rituximab maintenance in patients with follicular lymphoma diagnosed between 2004 and 2007 in the United States. Cancer. 2014;120(12):1830–7. doi:10.1002/cncr.28659.
Federico M, Luminari S, Dondi A, Tucci A, Vitolo U, Rigacci L, Di Raimondo F, Carella AM, Pulsoni A, Merli F, Arcaini L, Angrilli F, Stelitano C, Gaidano G, Dell’olio M, Marcheselli L, Franco V, Galimberti S, Sacchi S, Brugiatelli M. R-CVP versus R-CHOP versus R-FM for the initial treatment of patients with advanced-stage follicular lymphoma: results of the FOLL05 trial conducted by the Fondazione Italiana Linfomi. J Clin Oncol. 2013;31(12):1506–13.
European Medicines Agency. Summary of product characteristics Levact®. Available at URL: http://www.aemps.gob.es/cima/pdfs/es/ft/72571/FT_72571.pdf.
Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grünhagen U, Losem C, Kofahl-Krause D, Heil G, Welslau M, Balser C, Kaiser U, Weidmann E, Dürk H, Ballo H, Stauch M, Roller F, Barth J, Hoelzer D, Hinke A, Brugger W, Study group indolent Lymphomas (StiL). Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203–10.
López-González A, Quero C, Cruz MA, Sabin P, Aguiar D, Gómez Codina J, Llanos M, Rodríguez D, Lavernia J, Provencio M. Survival analysis of follicular lymphoma in a national registry with over a thousand patients: Impact by treatment groups. (abstract 3605). European Cancer Congress 2013. Amsterdam, Netherlands 2013, september 27th-October 1st. Available at URL: http://www.poster-submission.com/search/sresult. Accessed 29th May 2014.
Salar A, Domingo-Doménech E, Panizo C, Nicolás C, Bargay J, Muntañola A, Canales M, Bello JL, Sancho JM, Tomás JF, Rodríguez MJ, Peñalver FJ, Grande C, Sánchez-Blanco JJ, Palomera L, Arranz R, Conde E, García M, García JF, Caballero D, Montalbán C, For the Grupo Español de Linfomas/Trasplante de Médula Ósea (GELTAMO). First-line response-adapted treatment with the combination of bendamustine and rituximab in patients with mucosa-associated lymphoid tissue lymphoma (MALT2008-01): a multicentre, single-arm, phase 2 trial. Lancet Haematology. 2014;1(3):e104–11.
Salles G, Seymour JF, Offner F, López-Guillermo A, Belada D, Xerri L, Feugier P, Bouabdallah R, Catalano JV, Brice P, Caballero D, Haioun C, Pedersen LM, Delmer A, Simpson D, Leppa S, Soubeyran P, Hagenbeek A, Casasnovas O, Intragumtornchai T, Fermé C, da Silva MG, Sebban C, Lister A, Estell JA, Milone G, Sonet A, Mendila M, Coiffier B, Tilly H. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377(9759):42–51.
van Oers MH, Van Glabbeke M, Giurgea L, Klasa R, Marcus RE, Wolf M, Kimby E, van t Veer M, Vranovsky A, Holte H, Hagenbeek A. Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin’s lymphoma: long-term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol. 2010;28(17):2853–8.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing 2014; Vienna. URL http://www.R-project.org/.
Therneau T. A Package for Survival Analysis in S. R package version 2. 2014; 37–7. http://CRAN.R-project.org/package=survival.
National Statistics Institute. Instituto Nacional de Estadística (INE). Tablas de mortalidad de la población de España 1991-2012. Available at URL: http://www.ine.es/jaxi/tabla.do?path=/t20/p319a/serie/p01/l0/&file=01001.px&type=pcaxis&L=0. Accessed on 1 August 2014.
Swenson WT, Wooldridge JE, Lynch CF, Forman-Hoffman VL, Chrischilles E, Link BK. Improved survival of follicular lymphoma patients in the United States. J Clin Oncol. 2005;23(22):5019–26.
Pettengell R, Donatti C, Hoskin P, Poynton C, Kettle PJ, Hancock B, Johnson S, Dyer MJ, Rule S, Walker M, Wild D. The impact of follicular lymphoma on health related quality of life. Ann Oncol. 2008;19:570–6.
Dewilde S, Woods B, Castaigne JG, Parker C, Dunlop W. Bendamustine-rituximab: a cost-utility analysis in first-line treatment of indolent non-Hodgkin’s lymphoma in England and Wales. J Med Econ. 2014;17(2):111–24.
López-Bastida J, Oliva J, Antoñanzas F, García-Altés A, Gisbert R, Mar J, Puig-Junoy J. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ. 2010;11(5):513–20.
General Council of Official Pharmaceutical Collegues Bot Plus 2.0. Available at URL: http://www.portalfarma.com. Accessed on 13 December 2013.
Royal Decree-Law 8-2010. Real Decreto-ley 8/2010, de 20 de mayo, por el que se adoptan medidas extraordinarias para la reducción del déficit público. Available at URL: http://www.boe.es/boe/dias/2010/05/24/pdfs/BOE-A-2010-8228.pdf. Accessed on 13 December 2013.
Ministry of Health. Ministerio de Sanidad, Servicios Sociales e Igualdad. Listado de medicamentos afectados por el RD-L 8/2010. Available at URL. http://www.msssi.gob.es/profesionales/farmacia/pdf/deduccionesMay2014.pdf. Accessed on 13 December 2013.
Oblikue Consulting. eSalud database. Available at URL: http://www.oblikue.com. Accessed on 13 December 2013.
Sacristán JA, Oliva J, Del Llano J, Prieto L, Pinto JL. ¿Qué es una tecnología sanitaria eficiente en España? [What is an efficient health technology in Spain?] Gac Sanit. 2002;16(4):334–43.
Olin RL, Kanetsky PA, Ten Have TR, Nasta SD, Schuster SJ, Andreadis C. Determinants of the optimal first-line therapy for follicular lymphoma: a decision analysis. Am. J. Hematol. 2010;85:255–60.
Soini EJ, Martikainen JA, Vihervaara V, Mustonen K, Nousiainen T. Economic evaluation of sequential treatments for follicular non-Hodgkin lymphoma. Clin Ther. 2012;34(4):915–925.e2.
Su W, Quon P, Whalen J, Ranganathan G, Wronski S, Mwamburi M, Knopf KB, Sterchele JA, Salvador CG, Stillman IO, Kadambi A. Cost-effectiveness analysis of bendamustine plus rituximab versus CHOP-R in treatment-naive patients with mantle cell (MCL) and indolent lymphomas (IL). J Clin Oncol. 2012;30(15):6553.
Castro Gómez AJ, López-Guillermo A, Rueda Domínguez A, Salar A, Varela Moreno C, Rubio-Terrés C. Análisis Coste-Efectividad del tratamiento de mantenimiento con rituximab en pacientes con linfoma folicular que responden a la terapia de inducción en primera línea. Rev Esp Salud Pública. 2012;86:163–76.
Greiner W, Weijnen T, Nieuwenhuizen M, Oppe S, Badia X, Busschbach J, Buxton M, Dolan P, Kind P, Krabbe P, Ohinmaa A, Parkin D, Roset M, Sintonen H, Tsuchiya A, de Charro F. A single European currency for EQ-5D health states. Results from a six-country study. Eur J Health Econ. 2003;4(3):222–31.
Greenhalgh J, Bagust A, Boland A, Blundell M, Oyee J, Beale S, Dundar Y, Hockenhull J, Proudlove C, Chu P. Rituximab for the first-line maintenance treatment of follicular non-Hodgkin’s lymphoma : a NICE single technology appraisal. Pharmacoeconomics. 2013;31(5):403–13.
Acknowledgements
The authors would like to acknowledge the reviewers from Applied Health Economics and Health Policy for their helpful comments provided during the review process.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was performed thanks to financial support from Mundipharma Pharmaceuticals, S.L. (Spain).
Conflict of interest
ES and IO are employed by PORIB, a consultant company specializing in health economic evaluations. JMC is employed by Mundipharma Pharmaceuticals. AR has received honoraria from Mundipharma Pharmaceuticals, for advocacy tasks related to the present work as well as investigational funds and lecture honoraria from Roche Pharma. AS and ALG have received honoraria from Mundipharma Pharmaceuticals for advocacy tasks related to the present work.
Contributions
ES, IO and JMC conceived the model. ES and IO developed the model and drafted the manuscript. AR, AS and ALG validated the model structure, provided the data related to clinical practice and reviewed and approved the final version of manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sabater, E., López-Guillermo, A., Rueda, A. et al. Cost-Effectiveness Analysis of Bendamustine Plus Rituximab as a First-Line Treatment for Patients with Follicular Lymphoma in Spain. Appl Health Econ Health Policy 14, 465–477 (2016). https://doi.org/10.1007/s40258-016-0243-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-016-0243-4