Abstract
Background
The comparative efficacy of biologics and small-molecule inhibitors in treating palmoplantar psoriasis (PP) and palmoplantar pustulosis (PPP) remains uncertain.
Objective
The aim was to perform a systematic review and network meta-analysis (NMA) to compare the efficacy of biologics and small-molecule inhibitors for the treatment of PP and PPP.
Methods
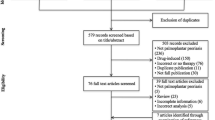
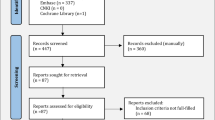
MEDLINE, Embase, and Cochrane Central Register of Controlled Trials were searched for eligible studies from inception to May 13, 2023. This NMA was conducted and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension Statement for Network Meta-Analyses guidelines. Frequentist random-effects models NMA was performed with the surface under the cumulative ranking curve calculated for ranking. Our primary outcome was the proportion of patients achieving a clear/minimal Palmoplantar Psoriasis/Pustulosis Physician Global Assessment score (PPPGA 0/1 or PPPPGA 0/1) response at 12–16 weeks. Secondary outcomes consisted of the percentage of overall improvement in palmoplantar score and of improvement ≥ 75%, at 12–16 weeks.
Results
The study comprised a total of 29 randomized controlled trials (RCTs), involving 4798 psoriasis patients with palmoplantar diseases. For PP, 16 RCTs with nine different treatments, including adalimumab, apremilast, bimekizumab, etanercept, guselkumab, infliximab, ixekizumab, secukinumab, and ustekinumab were included for the analysis. In the NMA of PP, secukinumab 300 mg ranked highest (odds ratio [OR] 33.50, 95% confidence interval [CI] 4.37–256.86) in achieving PPPGA 0/1, followed by guselkumab 100 mg (OR 18.68, 95% CI 10.07–34.65). In the case of PPP, seven RCTs with six treatments, including apremilast, etanercept, guselkumab, imsidolimab, spesolimab, and ustekinumab, were included for the analysis. In the NMA of PPP, although no treatment demonstrated a significant difference compared to placebo in achieving PPPPGA 0/1, guselkumab 100 mg showed the greatest statistically significant improvement in the palmoplantar score (weighted mean difference 31.73, 95% CI 19.89–43.57) as a secondary outcome.
Conclusion
Among all available biologics and small-molecule inhibitors, secukinumab 300 mg and guselkumab 100 mg had the most favorable efficacy in treating PP and PPP, respectively.






Similar content being viewed by others
References
Timotijevic ZS, Trajkovic G, Jankovic J, Relic M, Doric D, Vukicevic D, et al. How frequently does palmoplantar psoriasis affect the palms and/or soles? A systematic review and meta-analysis. Postepy Dermatol Alergol. 2019;36(5):595–603. https://doi.org/10.5114/ada.2019.89508.
Brunasso AM, Puntoni M, Aberer W, Delfino C, Fancelli L, Massone C. Clinical and epidemiological comparison of patients affected by palmoplantar plaque psoriasis and palmoplantar pustulosis: a case series study. Br J Dermatol. 2013;168(6):1243–51. https://doi.org/10.1111/bjd.12223.
Terui T, Kobayashi S, Okubo Y, Murakami M, Hirose K, Kubo H. Efficacy and safety of guselkumab, an anti-interleukin 23 monoclonal antibody, for palmoplantar pustulosis: a randomized clinical trial. JAMA Dermatol. 2018;154(3):309–16. https://doi.org/10.1001/jamadermatol.2017.5937.
Twelves S, Mostafa A, Dand N, Burri E, Farkas K, Wilson R, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143(3):1021–6. https://doi.org/10.1016/j.jaci.2018.06.038.
Chung J, Callis Duffin K, Takeshita J, Shin DB, Krueger GG, Robertson AD, et al. Palmoplantar psoriasis is associated with greater impairment of health-related quality of life compared with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2014;71(4):623–32. https://doi.org/10.1016/j.jaad.2014.04.063.
Bissonnette R, Poulin Y, Bolduc C, Maari C, Provost N, Syrotuik J, et al. Etanercept in the treatment of palmoplantar pustulosis. J Drugs Dermatol. 2008;7(10):940–6.
de Waal AC, van de Kerkhof PC. Pustulosis palmoplantaris is a disease distinct from psoriasis. J Dermatol Treat. 2011;22(2):102–5. https://doi.org/10.3109/09546631003636817.
Adisen E, Tekin O, Gulekon A, Gurer MA. A retrospective analysis of treatment responses of palmoplantar psoriasis in 114 patients. J Eur Acad Dermatol Venereol. 2009;23(7):814–9. https://doi.org/10.1111/j.1468-3083.2009.03197.x.
Spencer RK, Jin JQ, Elhage KG, Davis MS, Hakimi M, Bhutani T, et al. Comparative efficacy of biologics and oral agents in palmoplantar psoriasis and palmoplantar pustulosis: a systematic review and network meta-analysis of randomized clinical trials. J Am Acad Dermatol. 2023. https://doi.org/10.1016/j.jaad.2023.04.043.
Kt S, Thakur V, Narang T, Dogra S, Handa S. Comparison of the efficacy and safety of apremilast and methotrexate in patients with palmoplantar psoriasis: a randomized controlled trial. Am J Clin Dermatol. 2021;22(3):415–23. https://doi.org/10.1007/s40257-021-00596-6.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. https://doi.org/10.7326/M14-2385.
Kuo L-T, Shao S-C, Chi C-C. Ten essential steps for performing a systematic review: a quick tutorial. Dermatol Sin. 2022;40(4):204–6. https://doi.org/10.4103/1027-8117.362992.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;18(343): d5928. https://doi.org/10.1136/bmj.d5928.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. https://doi.org/10.1136/bmj.315.7109.629.
Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3(2):98–110. https://doi.org/10.1002/jrsm.1044.
Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7–8):932–44. https://doi.org/10.1002/sim.3767.
Kwon HH, Park HY, Choi SC, Bae Y, Kang C, Jung JY, et al. Combined fractional treatment of acne scars involving non-ablative 1,550-nm erbium-glass laser and micro-needling radiofrequency: a 16-week prospective, randomized split-face study. Acta Derm Venereol. 2017;97(8):947–51. https://doi.org/10.2340/00015555-2701.
Bissonnette R, Poulin Y, Guenther L, Lynde CW, Bolduc C, Nigen S. Treatment of palmoplantar psoriasis with infliximab: a randomized, double-blind placebo-controlled study. J Eur Acad Dermatol Venereol. 2011;25(12):1402–8. https://doi.org/10.1111/j.1468-3083.2011.03984.x.
Bissonnette R, Pariser DM, Wasel NR, Goncalves J, Day RM, Chen R, et al. Apremilast, an oral phosphodiesterase-4 inhibitor, in the treatment of palmoplantar psoriasis: results of a pooled analysis from phase II PSOR-005 and phase III Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis (ESTEEM) clinical trials in patients with moderate to severe psoriasis. J Am Acad Dermatol. 2016;75(1):99–105. https://doi.org/10.1016/j.jaad.2016.02.1164.
Bissonnette R, Haydey R, Rosoph LA, Lynde CW, Bukhalo M, Fowler JF, et al. Apremilast for the treatment of moderate-to-severe palmoplantar psoriasis: results from a double-blind, placebo-controlled, randomized study. J Eur Acad Dermatol Venereol. 2018;32(3):403–10. https://doi.org/10.1111/jdv.14647.
Blauvelt A, Papp KA, Griffiths CE, Randazzo B, Wasfi Y, Shen YK, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–17. https://doi.org/10.1016/j.jaad.2016.11.041.
Study of tumor necrosis factor receptor fusion protein etanercept (Enbrel) in psoriasis of the hands and/or feet. [cited; Available from:]. Available from: https://clinicaltrials.gov/ct2/show/NCT00585650?term=NCT00585650&draw=2&rank=1. Access date May 2023.
Elewski B, Rich P, Crowley J, Foley P, Wu T, Reyes-servin O, Poulin Y. Risankizumab profile in nail, scalp, and palmoplantar psoriasis: efficacy and safety at 52 weeks in an integrated analysis of patients with moderate-to-severe plaque psoriasis.In: 24th World Congress of Dermatology. Milan, Italy.
Foley P, Gordon K, Griffiths CEM, Wasfi Y, Randazzo B, Song M, et al. Efficacy of guselkumab compared with adalimumab and placebo for psoriasis in specific body regions: a secondary analysis of 2 randomized clinical trials. JAMA Dermatol. 2018;154(6):676–83. https://doi.org/10.1001/jamadermatol.2018.0793.
Gottlieb A, Sullivan J, van Doorn M, Kubanov A, You R, Parneix A, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76(1):70–80. https://doi.org/10.1016/j.jaad.2016.07.058.
Hassanandani T, Panda M, Jena AK, Raj C. Methotrexate monotherapy versus methotrexate and apremilast combination therapy in the treatment of palmoplantar psoriasis: A prospective, randomised, assessor-blinded, comparative study. Indian J Dermatol Venereol Leprol. 2023;89(2):213–20. https://doi.org/10.25259/IJDVL_843_2021.
Leonardi C, Langley RG, Papp K, Tyring SK, Wasel N, Vender R, et al. Adalimumab for treatment of moderate to severe chronic plaque psoriasis of the hands and feet: efficacy and safety results from REACH, a randomized, placebo-controlled, double-blind trial. Arch Dermatol. 2011;147(4):429–36. https://doi.org/10.1001/archdermatol.2010.384.
Menter A, Warren RB, Langley RG, Merola JF, Kerr LN, Dennehy EB, et al. Efficacy of ixekizumab compared to etanercept and placebo in patients with moderate-to-severe plaque psoriasis and non-pustular palmoplantar involvement: results from three phase 3 trials (UNCOVER-1, UNCOVER-2 and UNCOVER-3). J Eur Acad Dermatol Venereol. 2017;31(10):1686–92. https://doi.org/10.1111/jdv.14237.
Papp KA, Blauvelt A, Bukhalo M, Gooderham M, Krueger JG, Lacour JP, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376(16):1551–60. https://doi.org/10.1056/NEJMoa1607017.
Paul C, Reich K, Gottlieb AB, Mrowietz U, Philipp S, Nakayama J, et al. Secukinumab improves hand, foot and nail lesions in moderate-to-severe plaque psoriasis: subanalysis of a randomized, double-blind, placebo-controlled, regimen-finding phase 2 trial. J Eur Acad Dermatol Venereol. 2014;28(12):1670–5. https://doi.org/10.1111/jdv.12359.
Papp K, Lebwohl M, Gottlieb A, Sebastian M, Langley R, Okubo Y, Wang M, Cioffi C, Staelens F, Reich K. Bimekizumab for the treatment of moderate to severe plaque psoriasis with scalp, nail and palmoplantar involvement through 52 weeks: post-hoc analysis from the BE VIVID phase 3 trial. SKIN J Cutan Med. 2021;5(1): s18. https://doi.org/10.25251/skin.5.supp.18.
Sigurgeirsson B, Kircik L, Nemoto O, Mikazans I, Haemmerle S, Thurston HJ, et al. Secukinumab improves the signs and symptoms of moderate-to-severe plaque psoriasis in subjects with involvement of hands and/or feet: subanalysis of a randomized, double-blind, placebo-controlled, phase 2 dose-ranging study. J Eur Acad Dermatol Venereol. 2014;28(8):1127–9. https://doi.org/10.1111/jdv.12343.
Bissonnette R, Nigen S, Langley RG, Lynde CW, Tan J, Fuentes-Duculan J, et al. Increased expression of IL-17A and limited involvement of IL-23 in patients with palmo-plantar (PP) pustular psoriasis or PP pustulosis; results from a randomised controlled trial. J Eur Acad Dermatol Venereol. 2014;28(10):1298–305. https://doi.org/10.1111/jdv.12272.
A study to evaluate the efficacy and safety of imsidolimab (ANB019) in adults with palmoplantar pustulosis (POPLAR). [cited; Available from:]. Available from: https://clinicaltrials.gov/ct2/show/NCT03633396?term=NCT03633396&draw=2&rank=1. Access date May 2023.
A study to test how effective and safe different doses of BI 655130 are in patients with a moderate to severe form of the skin disease palmoplantar pustulosis [cited; Available from:]. Available from: https://clinicaltrials.gov/ct2/show/NCT04015518?term=NCT04015518&draw=2&rank=1. Access date May 2023.
Mrowietz U, Burden AD, Pinter A, Reich K, Schakel K, Baum P, et al. Spesolimab, an anti-interleukin-36 receptor antibody, in patients with palmoplantar pustulosis: results of a phase IIA, multicenter, double-blind, randomized, placebo-controlled pilot study. Dermatol Ther (Heidelb). 2021;11(2):571–85. https://doi.org/10.1007/s13555-021-00504-0.
Terui T, Kobayashi S, Okubo Y, Murakami M, Zheng R, Morishima H, et al. Efficacy and safety of guselkumab in japanese patients with palmoplantar pustulosis: a phase 3 randomized clinical trial. JAMA Dermatol. 2019;155(10):1153–61. https://doi.org/10.1001/jamadermatol.2019.1394.
Terui T, Okubo Y, Kobayashi S. 33142 efficacy and safety of apremilast for the treatment of Japanese patients with palmoplantar pustulosis: results from a phase 2, randomized, placebo-controlled study. J Am Acad Dermatol. 2022;87(3, Supplement):AB50. https://doi.org/10.1016/j.jaad.2022.06.233.
U.S. Food and Drug Administration. Cosentyx. U.S. Food and Drug Administration website. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125504s000lbl.pdf. Access date Jun 2023.
Mosca M, Hong J, Hadeler E, Hakimi M, Liao W, Bhutani T. The role of IL-17 cytokines in psoriasis. Immunotargets Ther. 2021;10:409–18. https://doi.org/10.2147/ITT.S240891.
Huang IH, Wu PC, Yang TH, Li H, Huang YT, Cheng YC, et al. Small molecule inhibitors and biologics in treating nail psoriasis: a systematic review and network meta-analysis. J Am Acad Dermatol. 2021;85(1):135–43. https://doi.org/10.1016/j.jaad.2021.01.024.
Sbidian E, Chaimani A, Garcia-Doval I, Doney L, Dressler C, Hua C, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2022;5(5):CD011535. https://doi.org/10.1002/14651858.CD011535.pub5.
Narcisi A, Valenti M, Cortese A, Toso F, Pavia G, Gargiulo L, et al. Anti-IL17 and anti-IL23 biologic drugs for scalp psoriasis: a single-center retrospective comparative study. Dermatol Ther. 2022;35(2): e15228. https://doi.org/10.1111/dth.15228.
Raposo I, Torres T. Palmoplantar psoriasis and palmoplantar pustulosis: current treatment and future prospects. Am J Clin Dermatol. 2016;17(4):349–58. https://doi.org/10.1007/s40257-016-0191-7.
Lee E, Zarei M, LaSenna C, Villada G, Romanelli P. Psoriasis targeted therapy: characterization of interleukin 17A expression in subtypes of psoriasis. J Drugs Dermatol. 2015;14(10):1133–6.
Galluzzo M, Talamonti M, Atzori L, Bardazzi F, Campanati A, Di Cesare A, et al. Secukinumab for the treatment of palmoplantar psoriasis: a 2-year, multicenter, real-life observational study. Expert Opin Biol Ther. 2022;22(4):547–54. https://doi.org/10.1080/14712598.2022.2029841.
Galluzzo M, D’Adamio S, Silvaggio D, Lombardo P, Bianchi L, Talamonti M. In which patients the best efficacy of secukinumab? Update of a real-life analysis after 136 weeks of treatment with secukinumab in moderate-to-severe plaque psoriasis. Expert Opin Biol Ther. 2020;20(2):173–82. https://doi.org/10.1080/14712598.2020.1708897.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Funding
None.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Ethics approval
Not applicable.
Consent to participate/publish
Not applicable.
Author contributions
All authors contributed to the study design. The search strategy development and literature search were performed by IHH and PCW. Data collection and interpretation were conducted by IHH, PCW, HYC, and YHH. Statistical analysis was performed by IHH. The manuscript was drafted by IHH and PCW and critically revised by all other authors. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, IH., Wu, PC., Chiu, HY. et al. Small-Molecule Inhibitors and Biologics for Palmoplantar Psoriasis and Palmoplantar Pustulosis: A Systematic Review and Network Meta-Analysis. Am J Clin Dermatol (2024). https://doi.org/10.1007/s40257-024-00849-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s40257-024-00849-0