Abstract
Background
External genital warts are caused by various subtypes of the human papilloma virus and spread through direct skin-to-skin contact. Approximately 1% of the US population have external genital warts. Although cantharidin has been used to treat external genital warts for decades, there are no US Food and Drug Administration-approved cantharidin products and no reliable or controlled sources of cantharidin available. VP-102 is a drug-device combination product containing cantharidin (0.7% w/v) in a single-use shelf-stable applicator.
Objective
The objective of this randomized, double-blind, vehicle-controlled, phase II clinical trial was to determine the optimal regimen for the treatment, safety, and efficacy of VP-102 in external genital warts.
Methods
The study was conducted in two parts. Part A was dose finding and Part B was performed following the completion of Part A for a safety and efficacy evaluation. Following completion of Part A, 6-h and 24-h VP-102 regimens under occlusion were selected to be evaluated in Part B.
Results
Pooled results from Part B and Part A of the 6-h and 24-h VP-102 treatment regimens showed that 36.7% and 33.3% of participants achieved complete clearance of all treatable external genital warts at the end of treatment vs 4.2% (p < 0.0048) and 0% (p < 0.0075) with the vehicle. Adverse events experienced by the VP-102-treated participants were consistent with the pharmacodynamic action of cantharidin as a vesicant and were primarily mild to moderate in severity. The most common adverse events included application-site vesicles, pain, and erythema. No participants discontinued the study because of adverse events and no serious adverse events were deemed treatment related.
Conclusions
The adverse event profile and efficacy of VP-102 under occlusion demonstrated in this study support the conclusion that a 6-h or up to 24-h exposure regimen represents an acceptable risk:benefit profile and justifies the conduct of a larger vehicle-controlled phase III study in external genital warts.
Clinical Trial Registration
NCT03981822, actual study start date: 25 June, 2019; actual primary completion date: 21 May, 2020; actual study completion date: 8 July, 2020.
Similar content being viewed by others
Approximately 1% of the US population have external genital warts. |
VP-102 is a drug-device combination product containing cantharidin (0.7% w/v) in a single-use shelf-stable applicator. |
The adverse event profile and efficacy of VP-102 under occlusion demonstrated in this study support the conclusion that a 6-h or up to 24-h exposure regimen represents an acceptable risk:benefit profile.The conduct of a larger vehicle-controlled phase III study in external genital warts is warranted. |
1 Introduction/Background
External genital warts (EGW) or condyloma acuminatum are caused by the human papilloma virus (HPV); types 6 and 11 being responsible for the majority of EGW cases. External genital warts are spread through direct skin-to-skin contact [1], impacting both men and women. Despite EGW being a common sexually transmitted infection [2], affecting approximately 1% of sexually active adults in the USA at any given time, HPV itself is not a nationally notifiable health condition [3, 4].
Human papilloma virus can remain latent in the skin, with EGW appearing or spreading at any time [5]. The incubation period from infection to appearance of clinical EGW ranges from 3 to 32 weeks [6]. The four morphologic types of EGW are cauliflower shaped, flat, smooth papular, and keratotic. External genital warts range in size from 1 mm to several inches in diameter and may appear in groups or individually. Once present, EGW may increase in number and/or size, but may also spontaneously regress; approximately 30% of patients experience regression within 4 months after appearance. Even when EGW spontaneously regress, the majority of EGW may recur within 3 months of infection [6].
External genital warts typically occur on the epidermis in the anogenital area, including the vulva, penis, groin, suprapubic skin, perineum, perianal, or mucosal surfaces. Patients may be asymptomatic, or may experience pruritus, burning, and pain [6]. In addition to physical symptoms, EGW can be associated with negative sexual feelings, causing significant psychological and emotional effects [7, 8].
While EGW can resolve spontaneously, treatment is recommended for patients experiencing symptoms such as itching, burning, or pain. Treatment should also be considered when there are concerns about spread or transmission [9, 10]. The goal of treatment is to eliminate visible EGW, as it is not possible to eliminate the underlying HPV infection. Treatment of EGW can be challenging with slow or no improvement and high recurrence rates [10, 11].
Most treatments focus on the destruction of EGW via physical or chemical means (e.g., electrocautery, chemical burning, cryotherapy, trichloroacetic acid, laser, or surgery). Available treatments also include compounded cantharidin, podophyllin, sinecatechins, and immune modulators such as imiquimod. Cantharidin, a vesicant that causes localized blistering, has been used to treat EGW for decades [12]. Despite this, cantharidin is not US Food and Drug Administration approved; therefore, formulations can only be obtained outside the USA or through compounding pharmacies [13]. The result is an absence of standardized optimized formulations and a lack of uniform manufacturing processes in accordance with Good Manufacturing Practices. Because of this, raw material sources of cantharidin and topical formulations vary in both consistency and availability. The various methods of application (e.g., wooden sticks, toothpicks, cotton swabs) lack uniformity and validation [13].
VP-102 is a drug/device product containing the active ingredient, cantharidin (0.7% w/v) in a sealed glass ampule within a single-use applicator. The solution containing cantharidin is slightly viscous and essentially free of visual particulates. The solution is manufactured and tested in conformance to current Good Manufacturing Practices. The safety and efficacy of VP-102 have been demonstrated in two phase III trials for the treatment of molluscum contagiosum in participants aged 2 years and older [14, 15]; however, the efficacy and safety profile in patients with EGW was unknown, and these studies were not done under occlusion. The objective of this phase II study was to determine the two best treatment regimens (Part A) to assess the safety and efficacy of VP-102 (Part B) under occlusion in healthy immunocompetent adult participants with EGW.
2 Methods
This phase II, double-blind, vehicle-controlled trial included two parts: Part A (dose finding) and Part B (safety and efficacy). Participants eligible for inclusion were healthy immunocompetent adults aged 18 years and older with no prior treatment for EGW 14 days prior to screening. Participants were required to have a wash-out period of 30 days for cantharidin, candida antigen, diphencyprone, dinitrochlorobenzene, squaric acid dibutyl ester, and any other immunomodulating treatment 30 days before the screening visit. Participants were excluded if they were systemically immunosuppressed, require or required systemic immunosuppressive or immunomodulatory medications during the study or in the 30 days prior to enrollment, or had a medical condition that could interfere with the study results or place the participant at undue risk (e.g., human immunodeficiency virus). Participants had to abstain from other EGW treatments, including the HPV vaccine, during the study period. The EGW count was to be ≥ 2 and ≤ 30 for each participant within the allowed treatment areas (i.e., within the medial thigh, supra-pubic area, and/or perianal area) at the time of visit 1. External genital warts must have been present for ≥4 weeks at the baseline visit and measure ≤ 8 mm in diameter each with a total wart area (i.e., all EGW combined) ≥ 10 mm2.
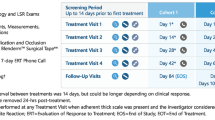
In Part A, increasing durations of skin exposure to VP-102 or vehicle were evaluated in three treatment groups, enrolled progressively and randomized in a 5:1 ratio (VP-102:vehicle) to each of the exposure regimens, 2-h, 6-h, and 24-h (Fig. 1). Randomization was performed such that each group contained a minimum of two subjects from each sex. External genital warts were treated with topical administration of VP-102 or vehicle every 21 days (visit 1/day 1, visit 2/day 21, visit 3/day 42, visit 4/day 63) until complete clearance or a maximum of four applications. The study drug was applied and covered with transparent surgical tape. Surgical tape was gently rubbed to maximize adherence to the treated area once the study drug had dried (approximately 2–5 min). An adhesive bandage could be used if needed for flexible areas or in some anatomical locations where surgical tape was not feasible. The tape and/or bandage was subsequently removed based on the assigned regimen. Enrollment began in the 2-h group, then proceeded into the 6-h group, and finally to the 24-h group. An in-person safety assessment was completed approximately 48 h after the first study drug application. The enrollment of participants into the next group was allowed upon completion of a review of the safety and tolerability data by a blinded Safety Review Panel, conducted after six participants in a designated group had completed the 48-h safety visit. An additional blinded safety review of treatment-emergent adverse events (TEAEs), focusing on moderate or severe intensity, was performed after all participants in the 24-h duration group completed the 48-h safety visit. The results from Part A were used to identify the best two dosing regimens that would be evaluated in Part B.
The objective of Part B was to identify the regimen with the best risk-to-benefit profile. Newly enrolled participants in Part B were randomized and stratified by sex to receive one of four treatment arms (VP-102 6-h exposure; VP-102 24-h exposure; vehicle 6-h exposure; vehicle 24-h exposure) in a 3:3:2:2 ratio (Fig. 1). As in Part A, EGWs were treated with topical administration of VP-102 or vehicle under occlusion for the designated exposure duration every 21 days (visit 1/day 1, visit 2/day 21, visit 3/day 42, visit 4/day 63) until complete clearance, or a maximum of four applications. The study drug was applied and covered with transparent surgical tape, which was subsequently removed based on the assigned regimen.
The primary efficacy endpoint was defined as the proportion of participants who experienced complete clearance of all treatable (baseline and new) EGW at the end of treatment (EOT) visit/day 84. Other efficacy endpoints included the proportion of participants achieving complete clearance of all treatable EGWs at visit 2/day 21, visit 3/day 42, visit 4/day 63, and percent change from baseline in the number of treatable (baseline and new) EGWs at visit 2/day 21, visit 3/day 42, visit 4/day 63, EOT visit/day 84.
The safety and tolerability of VP-102 in participants with EGW were evaluated by assessment of adverse events (AEs)/TEAEs including expected local skin reactions (LSRs). Participants were provided a take-home guide that included information about possible LSRs. In Part A and Part B, AEs were assessed via telephone at 24 h, 7 days, and 14 days after study drug administration. During Part A, AEs were also assessed with an in-person visit 48 h after visit 1 as previously described.
2.1 Statistical Analysis
No formal power calculations were performed for this study. The study was to enroll approximately 18 participants in Part A and approximately 90 participants in Part B. Data from Part A were pooled with Part B where applicable. All randomized participants were evaluated in the intent-to-treat population. If a participant discontinued the study prior to day 84, they were analyzed as failing to achieve complete clearance at day 84 and not replaced. If a participant discontinued the study drug but remained in the study, the participant was analyzed as a treatment failure as well. Treatment comparisons were made separately for each regimen (i.e., VP-102 6-h exposure vs vehicle 6-h exposure, and VP-102 24-h exposure vs vehicle 24-h exposure). Continuous variables were summarized with means, standard deviations, medians, minimums, and maximums. Categorical variables were summarized by counts and percent of participants in corresponding categories. Where appropriate, 95% confidence intervals were included. Missing values were not considered for percent calculations, unless stated otherwise. In those cases, footnotes specified the percent basis. The primary efficacy endpoint was complete clearance of all treatable EGW at EOT (day 84). The Cochran–Mantel–Haenszel test was used for treatment comparisons for complete clearance. A restricted maximum likelihood-based repeated-measures approach, using a mixed-effect model repeat measurement, was used to analyze percent change from baseline in the number of warts. The mixed-effect model repeat measurement included the fixed categorical effects of treatment, gender, visit, and treatment-by-visit interaction as well as the continuous fixed covariate of the baseline wart count. An unstructured (co)variance structure shared across treatment groups was used to model the within-subject errors. The least-squares means from the mixed-effect model repeat measurement were used for analysis of treatment comparisons. A post-hoc analysis comparing all VP-102-exposed participants to all vehicle-exposed participants was performed by pooling the 6-h and 24-h VP-102 regimens and pooling the 6-h and 24-h vehicle regimens. All analyses and tabulations were performed using SAS version 9.3 or higher and validated by an independent programmer upon completion.
The study was conducted in accordance with Good Clinical Practice as required by the International Council on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human use guidelines and in accordance with country-specific laws and regulations governing clinical studies of investigational products. An information and consent form approved by a study site’s institutional review board was signed by the participants or legal representative and the Investigator before enrollment of participants and was required by the sponsor prior to shipment of the study drug.
3 Results
3.1 Baseline Demographics and EGW Medical Histories
Eighteen participants were enrolled into Part A of the study, six in each of the three treatment regimens. Six participants who received the 6-h regimen and six participants who received the 24-h regimen from Part A were pooled with Part B. There were 87 newly enrolled participants in Part B. Therefore, the intent-to-treat and safety populations evaluated a total of 99 participants (pooled Part B and A).
Demographic details can be found in Table 1. Overall, across treatment groups, the mean age was 36 years, with a range of 25–59 years. Most participants were male (57.6% vs 42.4% female), White (73.7%); not Hispanic or Latino (85.9%) with a Fitzpatrick skin type III (37.4%) or IV (20.2%). Most participants (73 participants; 73.7%) in the study had between two and ten EGW at baseline; six participants (6.1%) had between 21 and 30 EGW at baseline. Fifty (50.5%) participants had EGW for a duration of < 1 year relative to other duration categories. Fifty-three (53.5%) participants had received prior EGW treatment, which was primarily freezing and “other” (various over-the-counter treatments, excision, laser, imiquimod).
3.2 Efficacy Outcomes
For both the 6-h and 24-h treatment regimens, the VP-102 groups showed a statistically significant separation from the vehicle in favor of VP-102 in complete clearance of EGW. In the 6-h treatment regimen, 36.7% (11/30) of participants receiving VP-102 had complete clearance of EGW at EOT visit/day 84 compared with 4.2% (1/24) of vehicle-treated participants (p = 0.0048, Fig. 2). In the 24-h VP-102 treatment regimen, 33.3% of participants (9/27) had complete clearance of EGW compared with no participants (0/18) in the vehicle group (p = 0.0075, Fig. 2) at EOT visit/day 84. Statistically significant differences in complete clearance of EGW were observed for both the 6-h and 24-h VP-102 regimens vs their respective vehicle treatment groups by visit 4/day 63 (p < 0.05 for both regimens vs vehicle) and were maintained through EOT visit/day 84 (p < 0.01 for both regimens vs vehicle) (Fig. 2).
Statistically significant reductions in the percentage change of EGW from baseline were observed for both VP-102 treatment regimens vs vehicle at visit 2/day 21 (p < 0.0001 at visit 2 for both 6-h and 24-h regimens) through the EOT visit/day 84 (p < 0.0001 at EOT visit for both 6-h and 24-h regimens) (see Fig. 3).
Mean percent change in external genital wart (EGW) count from baseline by study visit (Pooled B and A, intent-to-treat population). EOT end of treatment, MMRM mixed-effect model repeat measurement. P-value is based on MMRM with gender, treatment, visit, treatment by visit interaction, and baseline wart count as factors. An unstructured covariance model was used. Degrees of freedom associated with the error term were computed using the Kenward-Rogers method. Mean percent change reported are least-squares means
When comparing the two treatment regimens (6-h and 24-h regimens) at the EOT visit/day 84, there were no statistically significant differences in complete clearance of EGWs between the two VP-102 treatment groups (p = 0.7893). Therefore, a post-hoc analysis was performed pooling the 6-h and 24-h regimens (VP-102 vs vehicle). For the Part B and A pooled intent-to-treat population at EOT visit/day 84, complete clearance of EGW (pooled VP-102 and vehicle exposures) was statistically significant in the VP-102 treatment group (35.1% 20/57; p = 0.0001) compared with the vehicle group (2.4%, 1/42).
3.3 Safety Outcomes
Most participants (82%, 81/99) reported AEs/TEAEs, the majority of which were reported as mild or moderate in severity. Severe TEAEs were reported in two participants in the VP-102 24-h treatment group, which were attributed to vesicles (n = 1) and pruritis (n = 1). Serious AEs were reported in three participants across all treatment groups: VP-102 6-h group (n = 1), vehicle 6-h group (n = 1), and vehicle 24-h group (n = 1). None was considered related to treatment with the study drug.
The majority of reported TEAEs were LSRs, all of which were considered related to the study drug. All participants who received VP-102 reported LSRs. The most frequently reported LSRs were application-site vesicles, application-site pain, application-site erythema, application-site pruritus, and application-site scab (Table 2).
Non-LSR TEAEs were deemed to be unrelated to the study drug. Treatment-emergent AEs occurring in ≥ 5% of participants are displayed in Table 2. No serious TEAEs were attributed to the study drug. No participant discontinued treatment because of an AE. There were no deaths during the study.
Across treatment groups, 71 of 99 participants completed the study (range of 66.7–75.0%). The most frequently reported reason for study discontinuation was loss to follow-up (n = 18). Six participants discontinued because of COVID-19-related reasons and four participants withdrew for other unknown reasons.
4 Discussion
Human papilloma virus is the most common sexually transmitted infection in the USA, and accounted for 775 million dollars in direct medical costs in 2018. [3] Despite vaccination availability for HPV types 6 and 11 (the most common causes of EGW), the condition remains a common health concern for sexually active individuals, with 1% of the US population infected with HPV with EGW present at any given time. [4] Current treatments have variable efficacy with high recurrence rates. [11] There is an unmet need for effective treatment to (1) relieve physical symptoms, (2) minimize the risk of autoinoculation, and (3) prevent transmission to partners. [16]
Historically, compounded cantharidin has been used to treat EGW without the support of large randomized controlled studies. In a 2018 single-center, small, randomized controlled pilot study of 12 women with approximately two EGW at baseline, compounded cantharidin with occlusive tape demonstrated superior efficacy and safety compared with trichloroacetic acid. When compounded cantharidin was applied to EGW far from mucosal and intertriginous areas, participants described a painless in-office application followed by some pain and blistering several hours later. All patients in the compounded cantharidin group achieved complete clearance of EGW and patient satisfaction was higher compared with trichloroacetic acid treatment. [1]
Access to compounded cantharidin in the USA is limited. Formulations are only available via importation or through compounding pharmacies for single-patient use. The result is the absence of standardized optimized formulations, dosing, and delivery. [13] The lack of Food and Drug Administration approval and availability and limitations with consistency and shelf stability present concerns with the widespread use of compounded cantharidin. VP-102 would be the first commercially manufactured, shelf-stable cantharidin product available for the treatment of EGW.
Overall, VP-102-treated and vehicle-treated participants in both treatment regimens (6-h and 24-h exposure durations) were similar in demographics and EGW medical histories. Compared to the vehicle, treatment with both VP-102 regimens resulted in statistically significantly higher rates of complete clearance of baseline and new EGW at EOT visit/day 84. Other efficacy measures, including change in EGW counts, favored use of VP-102. It is possible that recurrence of EGW could occur during or after the study given that HPV can remain latent in the skin and appear or spread at any time. [1, 6]
Treatment-emergent AEs for VP-102-treated participants were similar for both treatment regimens (6-h and 24-h exposure durations). Local skin reactions were expected owing to the pharmacodynamic action of cantharidin as a vesicant. Overall, VP-102 was well tolerated as evidenced by discontinuations; no participants discontinued because of AEs and there were no serious AEs reported due to the study drug.
This phase II study demonstrated the safety and efficacy of VP-102 in the treatment of EGW. The 6-h and 24-h duration periods had similar safety and efficacy outcomes, suggesting that both exposure durations are efficacious and safe for the treatment of EGW.
4.1 Study Limitations
This was a small study and the results will be used to design a larger scale study. This phase II trial was impacted by the COVID-19 pandemic. Because of logistical and/or scheduling difficulties related to the COVID-19 pandemic during the study, six participants discontinued because of COVID-19-related reasons.
5 Conclusions
VP-102 was safe and well tolerated in this adult subject population with EGW. For efficacy endpoints analyzed, VP-102 was effective in attaining complete clearance of EGW and reducing the number of EGW in this subject population, demonstrating statistical significance vs vehicle. Efficacy results were comparable between the VP-102 6-h and 24-h treatment groups at day 84 (EOT). Both the VP-102 6-h and 24-h treatment groups presented comparable and favorable safety profiles, with most AEs being LSRs related to the mechanism of action of cantharidin, and the conduct of a larger, vehicle-controlled phase III study is warranted.
References
Recanati MA, Kramer KJ, Maggio JJ, Chao CR. Cantharidin is superior to trichloroacetic acid for the treatment of non-mucosal genital warts: a pilot randomized controlled trial. Clin Exp Obstet Gynecol. 2018;45(3):383–6.
Patel H, Wagner M, Singhal P, Kothari S. Systematic review of the incidence and prevalence of genital warts. BMC Infect Dis. 2013;25(13):39.
Centers for Disease Control and Prevention. Sexually transmitted infections (STIs) are very common and costly to the nation's health and economy. Sexually transmitted diseases (STDs) 2020. 25 January 2021. https://www.cdc.gov/std/statistics/prevalence-2020-at-a-glance.htm. Accessed 24 Apr 2021.
Centers for Disease Control and Prevention. Genital HPV infection: fact sheet. 19 January 2021. https://www.cdc.gov/std/hpv/stdfact-hpv.htm. Accessed 1 Jul 2021.
Leman JA, Benton EC. Verrucas. Am J Clin Dermatol. 2000;1(3):143–9.
Yanofsky VR, Patel RV, Goldenberg G. Genital warts: a comprehensive review. J Clin Aesthet Dermatol. 2012;5(6):25–36.
Beutner KR, Reitano MV, Richwald GA, Wiley DJ, Warts AEPoEG. external genital warts: report of the American Medical Association consensus conference. Clin Infect Dis. 1998;27(4):796–806.
Dediol I, Buljan M, Vurnek-? Ivkovi? M, Bulat V, itum M. Psychological burden of anogenital warts. J Eur Acad Dermatol Venereol. 2009;23(9):1035–8.
Allen AL, Siegfried EC. The natural history of condyloma in children. J Am Acad Dermatol. 1998;39(6):951–5.
Gilson R, Nugent D, Werner R, Ballesteros J, Ross J. 2019 IUSTI-Europe guideline for the management of anogenital warts. J Eur Acad Dermatol Venereol. 2020;34(8):1644–53.
Scheinfeld N. Update on the treatment of genital warts. Dermatol Online J. 2013;19(6):18559.
Epstein JH, Epstein WL. Cantharidin treatment of digital and periungual warts. Calif Med. 1960;93(1):11.
Del Rosso JQ, Kircik L. Topical cantharidin in the management of molluscum contagiosum: preliminary assessment of an ether-free, pharmaceutical-grade formulation. J Clin Aesthet Dermatol. 2019;12(2):27–30.
Eichenfield LF, McFalda W, Brabec B, Siegfried E, Kwong P, McBride M, et al. Safety and efficacy of VP-102, a proprietary, drug-device combination product containing cantharidin, 0.7%(w/v), in children and adults with molluscum contagiosum: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156(12):1315–23.
Eichenfield LF, Siegfried E, Kwong P, McBride M, Rieger J, Glover D, et al. Pooled results of two randomized phase III trials evaluating VP-102, a drug-device combination product containing cantharidin 0.7%(w/v) for the treatment of nolluscum contagiosum. Am J Clin Dermatol. 2021;22(2):257–65.
Markowitz LE, Liu G, Hariri S, Steinau M, Dunne EF, Unger ER. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics. 2016;137(3):e20151968.
Acknowledgements
We kindly acknowledge all of the participants and researchers that made this trial possible. We thank Mark McBride, PhD at Instat Clinical Research for his assistance with statistical methodology and analysis. We also thank Jessica McLin, PhD and Suzanne Silvers at Versant Learning Solutions for their assistance in finalizing the manuscript for submission, as well as completing the figures and formatting the tables.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Verrica Pharmaceuticals Inc. provided funding for the trial, as well as support for manuscript writing and figure creation.
Conflicts of interest/Competing interests
Drs. Guenthner, McFalda, and Tate received funding for clinical trials. Drs. Rieger and Glover are employees of PBM Capital Group. Dr. Andres, Ms. Willson, and Ms. Rumney are employees of Verrica Pharmaceuticals Inc. Dr. Olivadoti was an employee of Verrica Pharmaceuticals Inc. Dr. Rosen is a consultant for Verrica in activities unrelated to this trial.
Ethics approval
The study was conducted in accordance with Good Clinical Practice (GCP) as required by the International Council on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human use (ICH) guidelines and in accordance with country-specific laws and regulations governing clinical studies of investigational products.
Consent to participate
An Information and Consent form approved by a study site’s institutional review board (IRB) was signed by the participants or legal representative and the Investigator before enrollment of participants.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated during and/or analyzed during the current study are not publicly available as they are proprietary and under review with the FDA.
Code availability
Not applicable.
Authors’ contributions
Concept and design: Willson, Rumney, Glover, Rieger. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: All authors. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Willson, Rumney. Obtained funding: Willson, Rumney. Administrative, technical, or material support: All Authors. Supervision: All Authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Guenthner, S., McFalda, W., Tate, M. et al. Phase II, Double-Blind, Vehicle-Controlled Study to Determine the Cantharidin Dose Regimen, Efficacy, Safety, and Tolerability of VP-102 in Subjects with External Genital Warts. Am J Clin Dermatol 22, 867–875 (2021). https://doi.org/10.1007/s40257-021-00635-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-021-00635-2