Abstract
Background
In recent years, an association between dipeptidyl peptidase-4 (DPP-4) inhibitors and bullous pemphigoid has been detected in pharmacovigilance studies in European and Asian countries; however, no pharmacovigilance data have been published yet in the USA.
Objective
The objective of this study was to examine the relationship between bullous pemphigoid and DPP-4 inhibitors and other oral diabetes mellitus medications in the FDA Adverse Event Reporting System (FAERS).
Methods
Case/non-case analyses were performed in the FAERS using data from 2006 to 2020 to examine the reporting odds ratio (ROR) signal for bullous pemphigoid for all classes of oral diabetes medications. These analyses were performed under multiple conditions to control for bias: (1) comparison to all other drugs in the FAERS; (2) comparison to other diabetes medications; and (3) comparison to all other diabetes medications where only a single agent was implicated.
Results
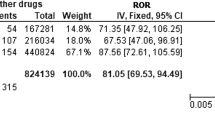
A statistically significant ROR for bullous pemphigoid was found for DPP-4 inhibitors under all conditions: (1) 109.79 (95% confidence interval [CI] 101.61–118.62); (2) 74.46 (95% CI 60.58–91.52); and (3) 35.94 (95% CI 27.91–46.28). A larger signal was seen for non-US Food and Drug Administration (FDA)-approved (anagliptin, vildagliptin, teneligliptin) vs FDA-approved DPP-4 inhibitors (alogliptin, linagliptin, saxagliptin, sitagliptin), likely because of an overestimation of the ROR for non-FDA-approved drugs. The largest signal was seen under conditions 1 and 2 with vildagliptin (1) 1022.83 (95% CI 909.45–1150.35) and (2) 158.84 (95% CI 127.01–198.66) followed by anagliptin (1) 628.63 (95% CI 221.36–1785.24) and (2) 60.64 (95% CI 20.98–175.26), alogliptin, teneligliptin, linagliptin, sitagliptin, and saxagliptin. Under condition 3, the largest signal was seen with linagliptin 122.25 (95% CI 93.96–159.07). Both metformin and the sulfonylureas had a significant ROR under condition 2 [3.42 (95% CI 3.01–3.89) and 2.07 (95% CI 1.66–2.57) respectively]; however, this association was not present under condition 3 as only confounded cases occurred, and a large majority of reported cases had concurrent exposure to a DPP-4 inhibitor.
Conclusions
Our findings support an association between DPP-4 inhibitors and bullous pemphigoid. This association was maintained under controls to limit bias and falsely elevated signal, including controlling for disease state and cases with multiple drug exposures. Non-FDA-approved DPP-4 inhibitors had a larger ROR compared with FDA-approved DPP-4 inhibitors, likely owing to fewer reported adverse effects overall for non-FDA-approved drugs in FAERS.

Similar content being viewed by others
References
Kasperkiewicz M, Zillikens D. The pathophysiology of bullous pemphigoid. Clin Rev Allergy Immunol. 2007;33(1–2):67–77.
Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381(9863):320–32.
Nishie W. Update on the pathogenesis of bullous pemphigoid: an autoantibody-mediated blistering disease targeting collagen XVII. J Dermatol Sci. 2014;73(3):179–86.
Tasanen K, Varpuluoma O, Nishie W. Dipeptidyl peptidase-4 inhibitor-associated bullous pemphigoid. Front Immunol. 2019;10:1238.
Lee SG, Lee HJ, Yoon MS, Kim DH. Association of dipeptidyl peptidase 4 inhibitor use with risk of bullous pemphigoid in patients with diabetes. JAMA Dermatol. 2019;155(2):172–7.
Persson MSM, Harman KE, Vinogradova Y, Langan SM, Hippisley-Cox J, Thomas KS, et al. Incidence, prevalence and mortality of bullous pemphigoid in England 1998–2017: a population-based cohort study. Br J Dermatol. 2021;184(1):68–77.
Liu SD, Chen WT, Chi CC. Association between medication use and bullous pemphigoid: a systematic review and meta-analysis. JAMA Dermatol. 2020;156(8):891–900.
Kridin K, Ludwig RJ. The growing incidence of bullous pemphigoid: overview and potential explanations. Front Med (Lausanne). 2018;5:220.
Fania L, Di Zenzo G, Didona B, Pilla MA, Sobrino L, Panebianco A, et al. Increased prevalence of diabetes mellitus in bullous pemphigoid patients during the last decade. J Eur Acad Dermatol Venereol. 2018;32(4):e153–4.
Jedlowski PM, Te CH, Segal RJ, Fazel MT. Cutaneous adverse effects of diabetes mellitus medications and medical devices: a review. Am J Clin Dermatol. 2019;20(1):97–114.
Le P, Chaitoff A, Misra-Hebert AD, Ye W, Herman WH, Rothberg MB. Use of antihyperglycemic medications in US adults: an analysis of the National Health and Nutrition Examination Survey. Diabetes Care. 2020;43(6):1227–33.
United Nations Department of Economic and Social Affairs PD. World population aging 2015. New York (NY): United Nations; 2015: p. 23–34.
Verheyden MJ, Bilgic A, Murrell DF. A systematic review of drug-induced pemphigoid. Acta Derm Venereol. 2020;10(15):224.
Arai M, Shirakawa J, Konishi H, Sagawa N, Terauchi Y. Bullous pemphigoid and dipeptidyl peptidase 4 inhibitors: a disproportionality analysis based on the Japanese Adverse Drug Event Report Database. Diabetes Care. 2018;41(9):e130–2.
Carnovale C, Mazhar F, Arzenton E, Moretti U, Pozzi M, Mosini G, et al. Bullous pemphigoid induced by dipeptidyl peptidase-4 (DPP-4) inhibitors: a pharmacovigilance-pharmacodynamic/pharmacokinetic assessment through an analysis of the vigibase(R). Expert Opin Drug Saf. 2019;18(11):1099–108.
Béné J, Moulis G, Bennani I, Auffret M, Coupe P, Babai S, et al. Bullous pemphigoid and dipeptidyl peptidase IV inhibitors: a case–noncase study in the French Pharmacovigilance Database. Br J Dermatol. 2016;175(2):296–301.
Molina-Guarneros JA, Sainz-Gil M, Sanz-Fadrique R, Garcia P, Rodriguez-Jimenez P, Navarro-Garcia E, et al. Bullous pemphigoid associated with the use of dipeptidil peptidase-4 inhibitors: analysis from studies based on pharmacovigilance databases. Int J Clin Pharm. 2020;42(2):713–20.
Reolid A, Munoz-Aceituno E, Rodriguez-Jimenez P, Gonzalez-Rojano E, Llamas-Velasco M, Fraga J, et al. Bullous pemphigoid associated with dipeptidyl peptidase-4 inhibitors: a case series and analysis of cases reported in the Spanish pharmacovigilance database. Int J Dermatol. 2020;59(2):197–206.
Douros A, Rouette J, Yin H, Yu OHY, Filion KB, Azoulay L. Dipeptidyl peptidase 4 inhibitors and the risk of bullous pemphigoid among patients with type 2 diabetes. Diabetes Care. 2019;42(8):1496–503.
Sim B, Fook-Chong S, Phoon YW, Koh HY, Thirumoorthy T, Pang SM, et al. Multimorbidity in bullous pemphigoid: a case-control analysis of bullous pemphigoid patients with age- and gender-matched controls. J Eur Acad Dermatol Venereol. 2017;31(10):1709–14.
Faillie JL. Case–non-case studies: principle, methods, bias and interpretation. Therapie. 2019;74(2):225–32.
Benzaquen M, Borradori L, Berbis P, Cazzaniga S, Valero R, Richard MA, et al. Dipeptidyl peptidase IV inhibitors, a risk factor for bullous pemphigoid: retrospective multicenter case-control study from France and Switzerland. J Am Acad Dermatol. 2018;78(6):1090–6.
Varpuluoma O, Forsti AK, Jokelainen J, Turpeinen M, Timonen M, Tasanen K, et al. Oral diabetes medications other than dipeptidyl peptidase 4 inhibitors are not associated with bullous pemphigoid: a Finnish nationwide case-control study. J Am Acad Dermatol. 2018;79(6):1034-8.e5.
Kridin K, Bergman R. Association of bullous pemphigoid with dipeptidyl-peptidase 4 inhibitors in patients with diabetes: estimating the risk of the new agents and characterizing the patients. JAMA Dermatol. 2018;154(10):1152–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding was used for the conduct of this study or the preparation of this article.
Conflict of interest
Patrick M. Jedlowski, Mahdieh F. Jedlowski, and Maryam T. Fazel have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by PJ. PJ, MJ, and MF wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jedlowski, P.M., Jedlowski, M.F. & Fazel, M.T. DPP-4 Inhibitors and Increased Reporting Odds of Bullous Pemphigoid: A Pharmacovigilance Study of the FDA Adverse Event Reporting System (FAERS) from 2006 to 2020. Am J Clin Dermatol 22, 891–900 (2021). https://doi.org/10.1007/s40257-021-00625-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-021-00625-4