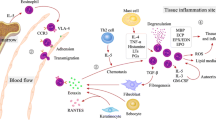
Abstract
Eosinophilic dermatoses encompass a broad spectrum of diseases of different etiologies hallmarked by eosinophilic infiltration of the skin and/or mucous membranes, with or without associated blood eosinophilia. The wide range of dermatological manifestations of this spectrum, including nodules and plaques, pustules, blisters, ulcers, and urticarial lesions, is reflected in a non-univocal classification system. We identified six groups of eosinophilic dermatoses based on the predominant anatomic level of involvement: (1) epidermal; (2) of the dermal–epidermal junction; (3) dermal; (4) of the hypodermis and muscle fascia; (5) of the pilosebaceous unit; and (6) vascular/perivascular. We review clinicopathologic features and management of diseases belonging to each group, particularly: (1) pemphigus herpetiformis and atopic dermatitis as prototypes of the epidermal group; (2) bullous pemphigoid as prototypic eosinophilic dermatosis of the dermal–epidermal junction; (3) eosinophilic cellulitis (Wells syndrome), hypereosinophilic syndromes, Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) syndrome, eosinophilic dermatosis of hematologic malignancy and chronic spontaneous urticaria as paradigmatic dermal eosinophilic dermatoses; (4) eosinophilic fasciitis as an eosinophilic dermatosis with predominant involvement of the hypodermis and muscle fascia; (5) eosinophilic pustular folliculitis as a model of the pilosebaceous unit involvement; and (6) granuloma faciale, angiolymphoid hyperplasia with eosinophilia, and eosinophilic granulomatosis with polyangiitis, belonging to the vascular/perivascular group.




Similar content being viewed by others
References
Peckruhn M, Elsner P, Tittelbach J. Eosinophilic dermatoses. J Dtsch Dermatol Ges. 2019;17:1039–51.
Leiferman KM, Peters MS. Eosinophil-related disease and the skin. J Allergy Clin Immunol Pract. 2018;6:1462–82 (e6).
Simon D, Simon HU. Eosinophilic disorders. J Allergy Clin Immunol. 2007;120:515.
Jablonska S, Chorzelski TP, Beutner EH, Chorzelska J. Herpetiform pemphigus, a variable pattern of pemphigus. Int J Dermatol. 1975;14:353–9.
Crotty C, Pittelkow M, Muller SA. Eosinophilic spongiosis: a clinicopathologic review of seventy-one cases. J Am Acad Dermatol. 1983;8:337–43.
Kasperkiewicz M, Kowalewski C, Jabłońska S. Pemphigus herpetiformis: from first description until now. J Am Acad Dermatol. 2014;70:780–7.
O’Toole EA, Mak LL, Guitart J, Woodley DT, Hashimoto T, Amagai M, et al. Induction of keratinocyte IL-8 expression and secretion by IgG autoantibodies as a novel mechanism of epidermal neutrophil recruitment in a pemphigus variant. Clin Exp Immunol. 2000;119:217–24.
Robinson ND, Hashimoto T, Amagai M, Chan LS. The new pemphigus variants. J Am Acad Dermatol. 1999;40:649–71.
de Graauw E, Beltraminelli H, Simon HU, Simon D. Eosinophilia in dermatologic disorders. Immunol Allergy Clin N Am. 2015;35:545–60.
Hossny E, Aboul-Magd M, Bakr S. Increased plasma eotaxin in atopic dermatitis and acute urticaria in infants and children. Allergy. 2001;56:996–1002.
Kiehl P, Falkenberg K, Vogelbruch M, Kapp A. Tissue eosinophilia in acute and chronic atopic dermatitis: a morphometric approach using quantitative image analysis of immunostaining. Br J Dermatol. 2001;145:720–9.
Leiferman KM, Ackerman SJ, Sampson HA, Haugen HS, Venencie PY, Gleich GJ. Dermal deposition of eosinophil-granule major basic protein in atopic dermatitis: comparison with onchocerciasis. N Engl J Med. 1985;313:282–5.
Roth N, Stadler S, Lemann M, Hosli S, Simon HU, Simon D. Distinct eosinophil cytokine expression patterns in skin diseases: the possible existence of functionally different eosinophil subpopulations. Allergy. 2011;66:1477–86.
Morshed M, Yousefi S, Stockle C, Simon HU, Simon D. Thymic stromal lymphopoietin stimulates the formation of eosinophil extracellular traps. Allergy. 2012;67:1127–37.
Simon D, Aeberhard C, Erdemoglu Y, Simon HU. Th17 cells and tissue remodeling in atopic and contact dermatitis. Allergy. 2014;69:125–31.
Simon D, Borradori L, Simon HU. Eosinophils as putative therapeutic targets in bullous pemphigoid. Exp Dermatol. 2017;26:1187–92.
de Graauw E, Sitaru C, Horn M, Borradori L, Yousefi S, Simon HU, et al. Evidence for a role of eosinophils in blister formation in bullous pemphigoid. Allergy Eur J Allergy Clin Immunol. 2017;72:1105–13.
Kridin K. Peripheral eosinophilia in bullous pemphigoid: prevalence and influence on the clinical manifestation. Br J Dermatol. 2018;179:1141–7.
Genovese G, Di Zenzo G, Cozzani E, Berti E, Cugno M, Marzano AV. New insights into the pathogenesis of bullous pemphigoid: 2019 update. Front Immunol. 2019;10:1506.
Rüdrich U, Gehring M, Papakonstantinou E, Illerhaus A, Engmann J, Kapp A, et al. Eosinophils are a major source of interleukin-31 in bullous pemphigoid. Acta Dermatovenereol. 2018;98:766–71.
Dresow SK, Sitaru C, Recke A, Oostingh GJ, Zillikens D, Gibbs BF. IgE autoantibodies against the intracellular domain of BP180. Br J Dermatol. 2009;160:429–32.
Cozzani E, Gasparini G, Di Zenzo G, Parodi A. Immunoglobulin E and bullous pemphigoid. Eur J Dermatol. 2018;28:440–8.
Messingham KN, Holahan HM, Frydman AS, Fullenkamp C, Srikantha R, Fairley JA. Human eosinophils express the high affinity IgE receptor, FceRI, in bullous pemphigoid. PLoS One. 2014;9:e107725.
Lin L, Hwang BJ, Culton DA, Li N, Burette S, Koller BH, et al. Eosinophils mediate tissue injury in the autoimmune skin disease bullous pemphigoid. J Investig Dermatol. 2018;138:1032–43.
Marzano AV, Tedeschi A, Berti E, Fanoni D, Crosti C, Cugno M. Activation of coagulation in bullous pemphigoid and other eosinophil-related inflammatory skin diseases. Clin Exp Immunol. 2011;165:44–50.
Marzano AV, Tedeschi A, Fanoni D, Bonanni E, Venegoni L, Berti E, et al. Activation of blood coagulation in bullous pemphigoid: role of eosinophils, and local and systemic implications. Br J Dermatol. 2009;160:266–72.
Tedeschi A, Marzano AV, Lorini M, Balice Y, Cugno M. Eosinophil cationic protein levels parallel coagulation activation in the blister fluid of patients with bullous pemphigoid. J Eur Acad Dermatol Venereol. 2015;29:813–7.
Cugno M, Marzano AV, Bucciarelli P, Balice Y, Cianchini G, Quaglino P, INVENTEP Study Group, et al. Increased risk of venous thromboembolism in patients with bullous pemphigoid. The INVENTEP (INcidence of VENous ThromboEmbolism in bullous Pemphigoid) study. Thromb Haemost. 2016;115:193–9.
Wells GC. Recurrent granulomatous dermatitis with eosinophilia. Trans St Johns Hosp Dermatol Soc. 1971;57:46–56.
Moossavi M, Mehregan DR. Wells’ syndrome: a clinical and histopathologic review of seven cases. Int J Dermatol. 2003;42:62–7.
Yu AM, Ito S, Leibson T, Lavi S, Fu LW, Weinstein M, Skotnicki SM. Pediatric Wells syndrome (eosinophilic cellulitis) after vaccination: a case report and review of the literature. Pediatr Dermatol. 2018;35:e262–e264264.
Caputo R, Marzano AV, Vezzoli P, Lunardon L. Wells syndrome in adults and children: a report of 19 cases. Arch Dermatol. 2006;142:1157–61.
Peckruhn M, Tittelbach J, Schliemann S, Elsner P. Life of lesions in eosinophilic cellulitis (Wells’ syndrome): a condition that may be missed at first sight. Am J Dermatopathol. 2015;37:e15–e1717.
Weins AB, Biedermann T, Weiss T, Weiss JM. Wells syndrome. J Dtsch Dermatol Ges. 2016;14:989–93.
Brasileiro LG, Abreu MAMM, Paschoal RS. Wells’ syndrome: the importance of differential diagnosis. An Bras Dermatol. 2019;94:370–2.
Ratzinger G, Zankl J, Zelger B. Wells syndrome and its relationship to Churg–Strauss syndrome. Int J Dermatol. 2013;52:949–54.
French LE, Shapiro M, Junkins-Hopkins JM, Wolfe JT, Rook AH. Eosinophilic fasciitis and eosinophilic cellulitis in a patient with abnormal circulating clonal T cells: increased production of interleukin 5 and inhibition by interferon alfa. J Am Acad Dermatol. 2003;49:1170–4.
Fujii K, Tanabe H, Kanno Y, Konishi K, Ohgou N. Eosinophilic cellulitis as a cutaneous manifestation of idiopathic hypereosinophilic syndrome. J Am Acad Dermatol. 2003;49:1174–7.
Räßler F, Lukács J, Elsner P. Treatment of eosinophilic cellulitis (Wells syndrome): a systematic review. J Eur Acad Dermatol Venereol. 2016;30:1465–79.
Karabudak O, Dogan B, Taskapan O, Harmanyeri Y. Eosinophilic cellulitis presented with semicircular pattern. J Dermatol. 2006;33:798–801.
Herr H, Koh JK. Eosinophilic cellulitis (Wells’ syndrome) successfully treated with low-dose cyclosporine. J Korean Med Sci. 2001;16:664–8.
Kim SH, Kwon JE, Kim HB. Successful treatment of steroid-dependent eosinophilic cellulitis with cyclosporine. Allergy Asthma Immunol Res. 2013;5:62–4.
Coelho de Sousa V, Laureano Oliveira A, Cardoso J. Successful treatment of eosinophilic cellulitis with dapsone. Dermatol Online J. 2016;22:13030.
Bokotas C, Kouris A, Stefanaki C, Sgotzou T, Christofidou E, Kontochristopoulos G. Wells syndrome: response to dapsone therapy. Ann Dermatol. 2014;26:541–2.
Moon SH, Shin MK. Bullous eosinophilic cellulitis in a child treated with dapsone. Pediatr Dermatol. 2013;30:e46–e4747.
Egeland Ø, Balieva F, Undersrud E. Wells syndrome: a case of successful treatment with omalizumab. Int J Dermatol. 2018;57:994–5.
Ogueta I, Spertino J, Deza G, Alcantara Luna S, Zaragoza Ninet V, Pujol RM, et al. Wells syndrome and chronic spontaneous urticaria: report of four cases successfully treated with omalizumab. J Eur Acad Dermatol Venereol. 2019;33:e388–e391391.
Coattrenec Y, Ibrahim LY, Harr T, Spoerl D, Jandus P. Long-term remission of Wells syndrome with omalizumab. J Investig Allergol Clin Immunol. 2020;30:58–9. https://doi.org/10.18176/jiaci.0436(Epub ahead of print).
Herout S, Bauer WM, Schuster C, Stingl G. Eosinophilic cellulitis (Wells syndrome) successfully treated with mepolizumab. JAAD Case Rep. 2018;4:548–50.
Ogbogu PU, Bochner BS, Butterfield JH, Gleich GJ, Huss-Marp J, Kahn JE, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009;124:1319–25 (e3).
Inayat F, O'Neill SS, Zafar F, Marupudi S, Vasim I. Idiopathic hypereosinophilic syndrome with cutaneous involvement: a comparative review of 32 cases. BMJ Case Rep. 2018;11(1):bcr2018227137.
Leiferman KM, Gleich GJ, Peters MS. Dermatologic manifestations of the hypereosinophilic syndromes. Immunol Allergy Clin N Am. 2007;27:415–41.
Valent P, Klion AD, Rosenwasser LJ, Arock M, Bochner BS, Butterfield JH, et al. ICON: eosinophil disorders. World Allergy Organ J. 2012;5:174–81.
Plötz SG, Hüttig B, Aigner B, Merkel C, Brockow K, Akdis C, et al. Clinical overview of cutaneous features in hypereosinophilic syndrome. Curr Allergy Asthma Rep. 2012;12:85–988.
Lefèvre G, Copin MC, Staumont-Sallé D, Avenel-Audran M, Aubert H, Taieb A, French Eosinophil Network, et al. The lymphoid variant of hypereosinophilic syndrome: study of 21 patients with CD3–CD4+ aberrant T-cell phenotype. Medicine (Baltimore). 2014;93:255–66.
Simon HU, Plötz SG, Dummer R, Blaser K. Abnormal clones of T cells producing interleukin-5 in idiopathic eosinophilia. N Engl J Med. 1999;341:1112–20.
Plötz SG, Simon HU, Darsow U, Simon D, Vassina E, Yousefi S, et al. Use of an anti-interleukin-5 antibody in the hypereosinophilic syndrome with eosinophilic dermatitis. N Engl J Med. 2003;349:2334–9.
Cusini M, Pizzati A, Genovese G, Muratori S, Schinco G, Berti E. Lymphocytic variant of hypereosinophilic syndrome presenting with polymorphic cutaneous manifestations and nonspecific histopathological findings. G Ital Dermatol Venereol. 2018. https://doi.org/10.23736/S0392-0488.18.06046-7(Epub ahead of print).
González Delgado P, de la Sen Fernández ML, Soriano Gomis V, Pérez Crespo M, Muñoz Ruiz C, Hernández NE. Cyclical hypereosinophilia with skin manifestations and a clonal T cell population. J Investig Allergol Clin Immunol. 2008;18:401–3.
Hayashi M, Kawaguchi M, Mitsuhashi Y, Suzuki T. Case of hypereosinophilic syndrome with cutaneous necrotizing vasculitis. J Dermatol. 2008;35:229–33.
Klion AD, Mejia R, Cowen EW, Dowdell KC, Dunleavy K, Fahle GA, et al. Chronic active Epstein–Barr virus infection: a novel cause of lymphocytic variant hypereosinophilic syndrome. Blood. 2013;121:2364–6.
d’Elbée JM, Parrens M, Mercié P, Longy Boursier M, Dieval C, de Mascarel A, et al. Hypereosinophilic syndrome: lymphocytic variant transforming into peripheral T-cell lymphoma with severe oral manifestations. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:e185–e190190.
Merlotto MR, Cantadori LO, Sakabe D, Miot HA. Case for diagnosis: erythroderma as manifestation of hypereosinophilic syndrome. An Bras Dermatol. 2018;93:451–3.
Sundaramurthi VL, Prabhavathy D, Somasundaram SV, Wahab AJ. Hypereosinophilic syndrome: cutaneous involvement as the sole manifestation. Indian J Dermatol. 2011;56:107–9.
Bogenrieder T, Griese DP, Schiffner R, Büttner R, Riegger GA, Hohenleutner U, et al. Wells’ syndrome associated with idiopathic hypereosinophilic syndrome. Br J Dermatol. 1997;137:978–82.
Kazmierowski JA, Chusid MJ, Parrillo JE, Fauci AS, Wolff SM. Dermatologic manifestations of the hypereosinophilic syndrome. Arch Dermatol. 1978;114:531–5.
Helbig G. Imatinib for the treatment of hypereosinophilic syndromes. Expert Rev Clin Immunol. 2018;14:163–70.
Curtis C, Ogbogu P. Hypereosinophilic syndrome. Clin Rev Allergy Immunol. 2016;50:240–51.
Rothenberg ME, Klion AD, Roufosse FE, Kahn JE, Weller PF, Simon HU, Mepolizumab HES Study Group, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358:1215–28.
Roufosse FE, Kahn JE, Gleich GJ, Schwartz LB, Singh AD, Rosenwasser LJ, et al. Long-term safety of mepolizumab for the treatment of hypereosinophilic syndromes. J Allergy Clin Immunol. 2013;131:461–7 (e1–5).
Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS). Semin Cutan Med Surg. 1996;15:250–7.
Chaiken BH, Goldberg BI, Segal JP. Dilantin sensitivity; report of a case of hepatitis with jaundice, pyrexia and exfoliative dermatitis. N Engl J Med. 1950;242:897–8.
Shiohara T, Mizukawa Y. Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): an update in 2019. Allergol Int. 2019;68:301–8.
Martínez-Cabriales SA, Rodríguez-Bolaños F, Shear NH. Drug reaction with eosinophilia and systemic symptoms (DReSS): how far have we come? Am J Clin Dermatol. 2019;20:217–36.
Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, Creamer D, RegiSCAR Study Group, et al. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071–80.
Watanabe H. Recent advances in drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms. J Immunol Res. 2018;2018:5163129.
Marzano AV, Borghi A, Cugno M. Adverse drug reactions and organ damage: the skin. Eur J Intern Med. 2016;28:17–24.
Ortonne N, Valeyrie-Allanore L, Bastuji-Garin S, Wechsler J, de Feraudy S, Duong TA, et al. Histopathology of drug rash with eosinophilia and systemic symptoms syndrome: a morphological and phenotypical study. Br J Dermatol. 2015;173:50–8.
Funck-Brentano E, Duong TA, Bouvresse S, Bagot M, Wolkenstein P, Roujeau JC, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246–52.
Weed RI. Exaggerated delayed hypersensitivity to mosquito bites in chronic lymphocytic leukemia. Blood. 1965;26:257–68.
Byrd JA, Scherschun L, Chaffins ML, Fivenson DP. Eosinophilic dermatosis of myeloproliferative disease: characterization of a unique eruption in patients with hematologic disorders. Arch Dermatol. 2001;137:1378–80.
Grandi V, Maglie R, Antiga E, Vannucchi M, Delfino C, Lastrucci I, et al. Eosinophilic dermatosis of hematologic malignancy: a retrospective cohort of 37 patients from an Italian center. J Am Acad Dermatol. 2019;81:246–9.
Maglie R, Antiga E, Vannucchi M, Del Bianco E, Bianchi B, Massi D, Caproni M. Bullous eruption in a patient with B-cell chronic lymphocytic leukemia: a diagnostic challenge. Int J Dermatol. 2017;56:1445–7.
Meiss F, Technau-Hafsi K, Kern JS, May AM. Eosinophilic dermatosis of hematologic malignancy: correlation of molecular characteristics of skin lesions and extracutaneous manifestations of hematologic malignancy. J Cutan Pathol. 2019;46:175–81.
Farber MJ, La Forgia S, Sahu J, Lee JB. Eosinophilic dermatosis of hematologic malignancy. J Cutan Pathol. 2012;39:690–5.
Jin A, Pousti BT, Savage KT, Mollanazar NK, Lee JB, Hsu S. Eosinophilic dermatosis of hematologic malignancy responding to dupilumab in a patient with chronic lymphocytic leukemia. JAAD Case Rep. 2019;5:815–7.
Asero R, Cugno M, Tedeschi A. Eosinophils in chronic urticaria: supporting or leading actors? World Allergy Organ J. 2009;2:213–7.
Yanase Y, Takahagi S, Hide M. Chronic spontaneous urticaria and the extrinsic coagulation system. Allergol Int. 2018;67:191–4.
Tedeschi A, Asero R, Lorini M, Marzano AV, Cugno M. Serum eotaxin levels in patients with chronic spontaneous urticaria. Eur Ann Allergy Clin Immunol. 2012;44:188–92.
Cugno M, Marzano AV, Tedeschi A, Fanoni D, Venegoni L, Asero R. Expression of tissue factor by eosinophils in patients with chronic urticaria. Int Arch Allergy Immunol. 2009;148:170–4.
Tedeschi A, Asero R, Marzano AV, Lorini M, Fanoni D, Berti E, Cugno M. Plasma levels and skin-eosinophil-expression of vascular endothelial growth factor in patients with chronic urticaria. Allergy. 2009;64:1616–22.
Kay AB, Ying S, Ardelean E, Mlynek A, Kita H, Clark P, et al. Elevations in vascular markers and eosinophils in chronic spontaneous urticarial weals with low-level persistence in uninvolved skin. Br J Dermatol. 2014;171:505–11.
Kolkhir P, Church MK, Altrichter S, Skov PS, Hawro T, Frischbutter S, et al. Eosinopenia, in chronic spontaneous urticaria, is associated with high disease activity, autoimmunity, and poor response to treatment. J Allergy Clin Immunol Pract. 2019. https://doi.org/10.1016/j.jaip.2019.08.025(Epub ahead of print).
Eberle JU, Radtke D, Nimmerjahn F, Voehringer D. Eosinophils mediate basophil-dependent allergic skin inflammation in mice. J Investig Dermatol. 2019;139:1957–65 (e2).
Shulman LE. Diffuse fasciitis with eosinophilia: a new syndrome? Trans Assoc Am Physicians. 1975;88:70–86.
Pinal-Fernandez I, Selva-O' Callaghan A, Grau JM. Diagnosis and classification of eosinophilic fasciitis. Autoimmun Rev. 2014;13:379–82.
Gomes I, Mathur SK, Espenshade BM, Mori Y, Varga J, Ackerman SJ. Eosinophil-fibroblast interactions induce fibroblast IL-6 secretion and extracellular matrix gene expression: implications in fibrogenesis. J Allergy Clin Immunol. 2005;116:796–804.
Mertens JS, Seyger MMB, Thurlings RM, Radstake TRDJ, de Jong EMGJ. Morphea and eosinophilic fasciitis: an update. Am J Clin Dermatol. 2017;18:491–512.
Lakhanpal S, Ginsburg WW, Michet CJ, Doyle JA, Moore SB. Eosinophilic fasciitis: clinical spectrum and therapeutic response in 52 cases. Semin Arthritis Rheum. 1988;17:221–31.
Fett N, Arthur M. Eosinophilic fasciitis: current concepts. Clin Dermatol. 2018;36:487–97.
Toquet C, Hamidou MA, Renaudin K, Jarry A, Foulc P, Barbarot S, et al. In situ immunophenotype of the inflammatory infiltrate in eosinophilic fasciitis. J Rheumatol. 2003;30:1811–5.
Mazori DR, Femia AN, Vleugels RA. Eosinophilic fasciitis: an updated review on diagnosis and treatment. Curr Rheumatol Rep. 2017;19:74.
De Clerck LS, Degryse HR, Wouters E, Van Offel JF, De Schepper AM, Martin JJ, et al. Magnetic resonance imaging in the evaluation of patients with eosinophilic fasciitis. J Rheumatol. 1989;16:1270–3.
Wright NA, Mazori DR, Patel M, Merola JF, Femia AN, Vleugels RA. Epidemiology and treatment of eosinophilic fasciitis: an analysis of 63 patients from 3 tertiary care centers. JAMA Dermatol. 2016;152:97–9.
Mertens JS, Zweers MC, Kievit W, Knaapen HK, Gerritsen M, Radstake TR, et al. High-dose intravenous pulse methotrexate in patients with eosinophilic fasciitis. JAMA Dermatol. 2016;152:1262–5.
Ise S, Ofuji S. Subcorneal pustular dermatosis: a follicular variant? Arch Dermatol. 1965;92(2):169–71.
Nakahigashi K, Doi H, Otsuka A, Hirabayashi T, Murakami M, Urade Y, et al. PGD2 induces eotaxin-3 via PPARγ from sebocytes: a possible pathogenesis of eosinophilic pustular folliculitis. J Allergy Clin Immunol. 2012;129:536–43.
Katoh M, Nomura T, Miyachi Y, Kabashima K. Eosinophilic pustular folliculitis: a review of the Japanese published works. J Dermatol. 2013;40:15–20.
Fujiyama T, Tokura Y. Clinical and histopathological differential diagnosis of eosinophilic pustular folliculitis. J Dermatol. 2013;40:419–23.
Hernández-Martín Á, Nuño-González A, Colmenero I, Torrelo A. Eosinophilic pustular folliculitis of infancy: a series of 15 cases and review of the literature. J Am Acad Dermatol. 2013;68:150–5.
Rosenthal D, LeBoit PE, Klumpp L, Berger TG. Human immunodeficiency virus-associated eosinophilic folliculitis: a unique dermatosis associated with advanced human immunodeficiency virus infection. Arch Dermatol. 1991;127:206–9.
Takamura S, Teraki Y. Eosinophilic pustular folliculitis associated with hematological disorders: a report of two cases and review of Japanese literature. J Dermatol. 2016;43:432–5.
Fukamachi S, Kabashima K, Sugita K, Kobayashi M, Tokura Y. Therapeutic effectiveness of various treatments for eosinophilic pustular folliculitis. Acta Derm Venereol. 2009;89:155–9.
Wigley JE. Eosinophilic granuloma? Sarcoid of Boeck. Proc R Soc Med. 1945;38:125–6.
Cesinaro AM, Lonardi S, Facchetti F. Granuloma faciale: a cutaneous lesion sharing features with IgG4-associated sclerosing diseases. Am J Surg Pathol. 2013;37:66–73.
Ortonne N, Wechsler J, Bagot M, Grosshans E, Cribier B. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002–9.
Lindhaus C, Elsner P. Granuloma faciale treatment: a systematic review. Acta Dermatovenereol. 2018;98:14–8.
Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969;81:1–14.
Tokat F, Lehman JS, Sezer E, Cetin ED, Ince U, Durmaz EO. Immunoreactivity of Wilms tumor 1 (WT1) as an additional evidence supporting hemangiomatous rather than inflammatory origin in the etiopathogenesis of angiolymphoid hyperplasia with eosinophilia. Dermatol Pract Concept. 2018;8:28–322.
Adler BL, Krausz AE, Minuti A, Silverberg JI, Lev-Tov H. Epidemiology and treatment of angiolymphoid hyperplasia with eosinophilia (ALHE): a systematic review. J Am Acad Dermatol. 2016;74:506–12 (e11).
Olsen TG, Helwig EB. Angiolymphoid hyperplasia with eosinophilia: a clinicopathologic study of 116 patients. J Am Acad Dermatol. 1985;12:781–96.
Marzano AV, Raimondo MG, Berti E, Meroni PL, Ingegnoli F. Cutaneous manifestations of ANCA-associated small vessels vasculitis. Clin Rev Allergy Immunol. 2017;53:428–38.
Furuta S, Iwamoto T, Nakajima H. Update on eosinophilic granulomatosis with polyangiitis. Allergol Int. 2019;68:430–6.
Chen KR, Carlson JA. Clinical approach to cutaneous vasculitis. Am J Clin Dermatol. 2008;9:71–92.
Marques CC, Fernandes EL, Miquelin GM, Colferai MMT. Cutaneous manifestations of Churg–Strauss syndrome: key to diagnosis. An Bras Dermatol. 2017;92:56–8.
Josselin-Mahr L, Werbrouck-Chiraux A, Garderet L, Cabane J. Efficacy of imatinib mesylate in a case of Churg–Strauss syndrome: evidence for the pathogenic role of a tyrosine kinase? Rheumatology (Oxford). 2014;53:378–9.
Erre GL, Pardini S, Cuccuru L, Taras L, Passiu G. Is there a role for imatinib mesylate in the treatment of eosinophilic granulomatosis with polyangiitis? Jt Bone Spine. 2015;82:72–3.
Simon D, Simon HU. Therapeutic strategies for eosinophilic dermatoses. Curr Opin Pharmacol. 2019;46:29–33.
Wakugawa M, Nakamura K, Hino H, Toyama K, Hattori N, Okochi H, et al. Elevated levels of eotaxin and interleukin-5 in blister fluid of bullous pemphigoid: correlation with tissue eosinophilia. Br J Dermatol. 2000;143:112–6.
Alsorori E, Kiss N, Medvecz M, Naqeshbandi AF, Bergler-Czop B, Alsarari I, et al. A case of granuloma faciale successfully treated with systemic dapsone. Dermatol Ther. 2019;8:e13162 (Epub ahead of print).
Montgomery JR, Chan LS. An unusual clinical presentation of pemphigus herpetiformis with marked response to dapsone. J Am Acad Dermatol. 2011;65:436–8.
Bozeman PM, Learn DB, Thomas EL. Inhibition of the human leukocyte enzymes myeloperoxidase and eosinophil peroxidase by dapsone. Biochem Pharmacol. 1992;44:553–63.
Günther C, Wozel G, Meurer M, Pfeiffer C. Up-regulation of CCL11 and CCL26 is associated with activated eosinophils in bullous pemphigoid. Clin Exp Immunol. 2011;166:145–53.
Buttgereit T, Bonnekoh H, Church MK, Bergmann KC, Siebenhaar F, Metz M. Effective treatment of a lymphocytic variant of hypereosinophilic syndrome with reslizumab. J Dtsch Dermatol Ges. 2019;17:1171–2.
Kuruvilla M. Treatment of hypereosinophilic syndrome and eosinophilic dermatitis with reslizumab. Ann Allergy Asthma Immunol. 2018;120:670–1.
Dávila González I, Moreno Benítez F, Quirce S. Benralizumab: a new approach for the treatment of severe eosinophilic asthma. J Investig Allergol Clin Immunol. 2019;29:84–93.
Oldhoff JM, Darsow U, Werfel T, Katzer K, Wulf A, Laifaoui J, et al. Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy. 2005;60:693–6.
Simon D, Yousefi S, Cazzaniga S, Bürgler C, Radonjic S, Houriet C, et al. Mepolizumab failed to affect bullous pemphigoid: a randomized, placebo-controlled, double-blind phase 2 pilot study. Allergy. 2019. https://doi.org/10.1111/all.13950(Epub ahead of print).
Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, EGPA Mepolizumab Study, et al. Team Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376:1921–32.
Faverio P, Bonaiti G, Bini F, Vaghi A, Pesci A. Mepolizumab as the first targeted treatment for eosinophilic granulomatosis with polyangiitis: a review of current evidence and potential place in therapy. Ther Clin Risk Manag. 2018;14:2385–96.
Kunsleben N, Rüdrich U, Gehring M, Novak N, Kapp A, Raap U. IL-31 induces chemotaxis, calcium mobilization, release of reactive oxygen species, and CCL26 in eosinophils, which are capable to release IL-31. J Investig Dermatol. 2015;135:1908–11.
Gibbs BF, Patsinakidis N, Raap U. Role of the pruritic cytokine IL-31 in autoimmune skin diseases. Front Immunol. 2019;10:1383.
Ruzicka T, Hanifin JM, Furue M, Pulka G, Mlynarczyk I, Wollenberg A, XCIMA Study Group, et al. Anti-interleukin-31 receptor A antibody for atopic dermatitis. N Engl J Med. 2017;376:826–35.
Silverberg JI, Pinter A, Pulka G, Poulin Y, Bouaziz JD, Wollenberg A, et al. Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J Allergy Clin Immunol. 2020;145:173–82.
Beck LA, Thaçi D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130–9.
Simpson EL, Flohr C, Eichenfield LF, Bieber T, Sofen H, Taïeb A, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol. 2018;78:863–71 (e11).
Wollenberg A, Howell MD, Guttman-Yassky E, Silverberg JI, Kell C, Ranade K, et al. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J Allergy Clin Immunol. 2019;143:135–41.
Hirano I, Dellon ES, Hamilton JD, Collins MH, Peterson K, Chehade M, et al. Efficacy of dupilumab in a phase 2 randomized trial of adults with active eosinophilic esophagitis. Gastroenterology. 2020;158:111–22 (e10).
Kaye A, Gordon SC, Deverapalli SC, Her MJ, Rosmarin D. Dupilumab for the treatment of recalcitrant bullous pemphigoid. JAMA Dermatol. 2018;154:1225–6.
Dworetzky SI, Hebrank GT, Archibald DG, Reynolds IJ, Farwell W, Bozik ME. The targeted eosinophil-lowering effects of dexpramipexole in clinical studies. Blood Cells Mol Dis. 2017;63:62–5.
Panch SR, Bozik ME, Brown T, Makiya M, Prussin C, Archibald DG, et al. Dexpramipexole as an oral steroid-sparing agent in hypereosinophilic syndromes. Blood. 2018;132:501–9.
O'Sullivan JA, Carroll DJ, Cao Y, Salicru AN, Bochner BS. Leveraging Siglec-8 endocytic mechanisms to kill human eosinophils and malignant mast cells. J Allergy Clin Immunol. 2018;141:1774–85 (e7).
Youngblood BA, Brock EC, Leung J, Falahati R, Bochner BS, Rasmussen HS, et al. Siglec-8 antibody reduces eosinophils and mast cells in a transgenic mouse model of eosinophilic gastroenteritis. JCI Insight. 2019;4(19):126219 (Epub ahead of print).
Marcus N, Smuel K, Almog M, Prais D, Straussberg R, Landau D, et al. Successful intravenous immunoglobulin treatment in pediatric severe DRESS syndrome. J Allergy Clin Immunol Pract. 2018;6:1238–42.
von Gunten S, Vogel M, Schaub A, Stadler BM, Miescher S, Crocker PR, et al. Intravenous immunoglobulin preparations contain anti-Siglec-8 autoantibodies. J Allergy Clin Immunol. 2007;119:1005–111.
Walker S, Wang C, Walradt T, Hong BS, Tanner JR, Levinsohn JL, et al. Identification of a gain-of-function STAT3 mutation (p.Y640F) in lymphocytic variant hypereosinophilic syndrome. Blood. 2016;12:948–51.
King B, Lee AI, Choi J. Treatment of hypereosinophilic syndrome with cutaneous involvement with the JAK inhibitors tofacitinib and ruxolitinib. J Investig Dermatol. 2017;137:951–4.
Bissonnette R, Papp KA, Poulin Y, Gooderham M, Raman M, Mallbris L, et al. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. Br J Dermatol. 2016;175(5):902–11.
Guttman-Yassky E, Silverberg JI, Nemoto O, Forman SB, Wilke A, Prescilla R, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J Am Acad Dermatol. 2019;80:913–21 (e9).
Guttman-Yassky E, Thaçi D, Pangan AL, Hong HC, Papp KA, Reich K, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2019 (Epub ahead of print)
Kim SR, Charos A, Damsky W, Heald P, Girardi M, King BA. Treatment of generalized deep morphea and eosinophilic fasciitis with the Janus kinase inhibitor tofacitinib. JAAD Case Rep. 2018;4:443–5.
Acknowledgements
The authors thank Raffaele Gianotti and Antonella Coggi for providing skin histopathology images and Claudia Menicanti for providing the clinical picture of angiolymphoid hyperplasia with eosinophilia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were received for the preparation of this article.
Conflict of interest
Angelo Valerio Marzano and Giovanni Genovese have no conflicts of interest that are directly relevant to the content of this article.
Rights and permissions
About this article
Cite this article
Marzano, A.V., Genovese, G. Eosinophilic Dermatoses: Recognition and Management. Am J Clin Dermatol 21, 525–539 (2020). https://doi.org/10.1007/s40257-020-00520-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-020-00520-4