Abstract
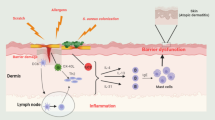
Atopic dermatitis (AD) is a chronic inflammatory skin disease that is characterized by complex pathophysiology involving both skin barrier dysfunction and aberrant type 2 inflammation/immune responses. AD can be a debilitating condition that drastically impairs quality of life, especially in patients with moderate-to-severe disease. Currently, topical therapies such as corticosteroids and non-steroidal immunomodulatory therapy provide limited efficacy for patients with moderate-to-severe AD; limitations include inadequate response, cutaneous toxicity from overuse, and poor tolerance due to stinging and burning. Historically, the development of targeted therapies has been challenging due to the complex and multifaceted etiology of AD. Recent progress in understanding the immunopathology of AD reinforces the development of newly targeted therapeutics. The successful launch of dupilumab, a monoclonal antibody targeting the interleukin (IL)-4α receptor subunit, for AD in 2017 spurred the development of a number of biologics targeting novel cytokine and receptor targets that are now in phase II and III of development. This review aims to explore the rationale behind these novel biological therapies and to summarize current clinical studies of these agents.
Similar content being viewed by others
References
Barbarot S, Auziere S, Gadkari A, Girolomoni G, Puig L, Simpson EL, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy. 2018;73(6):1284–93.
Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22(2):125–37.
Brunner PM, Silverberg JI, Guttman-Yassky E, Paller AS, Kabashima K, Amagai M, et al. Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J Invest Dermatol. 2017;137(1):18–25.
Silverberg JI. Atopic dermatitis treatment: current state of the art and emerging therapies. Allergy Asthma Proc. 2017;38(4):243–9.
Kim JE, Kim JS, Cho DH, Park HJ. Molecular mechanisms of cutaneous inflammatory disorder: atopic dermatitis. Int J Mol Sci. 2016;17(8):1234.
Guttman-Yassky E, Bissonnette R, Ungar B, Suarez-Farinas M, Ardeleanu M, Esaki H, et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143(1):155–72.
Klonowska J, Gleń J, Nowicki RJ, Trzeciak M. New cytokines in the pathogenesis of atopic dermatitis-new therapeutic targets. Int J Mol Sci. 2018;19(10):3086.
Noda S, Suarez-Farinas M, Ungar B, Kim SJ, de Guzman SC, Xu H, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015;136(5):1254–64.
Czarnowicki T, He H, Krueger JG, Guttman-Yassky E. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143(1):1–11.
DUPIXENT® (dupilumab) Injection. Package insert. Regeneron Pharmaceuticals, Inc. Tarrytown, NY2019.
Sanofi. FDA Approves Dupixent® (dupilumab) for moderate-to-severe atopic dermatitis in adolescents. 2019.
Silverberg JI, Kantor R. The role of Interleukins 4 and/or 13 in the pathophysiology and treatment of atopic dermatitis. Dermatol Clin. 2017;35(3):327–34.
Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–488.
Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–303.
Deleuran M, Thaci D, Beck LA, de Bruin-Weller M, Blauvelt A, Forman S, et al. Dupilumab shows long-term safety and efficacy in moderate-to-severe atopic dermatitis patients enrolled in a phase 3 open-label extension study. J Am Acad Dermatol. 2019;82(2):377–88.
Wollenberg A, Beck LA, Blauvelt A, Simpson EL, Chen Z, Chen Q, et al. Laboratory safety of dupilumab in moderate-to-severe atopic dermatitis: results from three phase III trials (LIBERTY AD SOLO 1, LIBERTY AD SOLO 2, LIBERTY AD CHRONOS). Br J Dermatol. 2019. https://doi.org/10.1111/bjd.18434.
Wollenberg A, Ariens L, Thurau S, van Luijk C, Seegräber M, de Bruin-Weller M. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab–clinical characteristics and treatment. J Allergy Clin Immunol Pract. 2018;6(5):1778–800.
Akinlade B, Guttman-Yassky E, de Bruin-Weller M, Simpson EL, Blauvelt A, Cork MJ, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. 2019;181(3):459–73.
Wollenberg A, Beck L, Blauvelt A, Simpson E, Chen Z, Chen Q, et al. Laboratory safety of dupilumab in moderate-to-severe atopic dermatitis: results from three phase III trials (LIBERTY AD SOLO 1, LIBERTY AD SOLO 2, LIBERTY AD CHRONOS). Br J Dermatol. 2019;. https://doi.org/10.1111/bjd.18434.
Dupilumab (Dupixent) for Asthma. JAMA. 2019;321(10):1000–1.
DUPIXENT® (dupilumab) Injection. Package insert. Regeneron Pharmaceuticals, Inc., Tarrytown, NY; Sanofi-Aventis U.S., Bridgewater, NJ; 2019. https://www.regeneron.com/sites/default/files/Dupixent_FPI.pdf. Accessed 20 Oct 2019.
Tsoi LC, Rodriguez E, Degenhardt F, Baurecht H, Wehkamp U, Volks N, et al. Atopic dermatitis is an IL-13-dominant disease with greater molecular heterogeneity compared to psoriasis. J Invest Dermatol. 2019;139(7):1480–9.
Zheng T, Oh MH, Oh SY, Schroeder JT, Glick AB, Zhu Z. Transgenic expression of interleukin-13 in the skin induces a pruritic dermatitis and skin remodeling. J Invest Dermatol. 2009;129(3):742–51.
Brandt EB, Sivaprasad U. Th2 cytokines and atopic dermatitis. J Clin Cell Immunol. 2011;2(3):110.
De Vuyst E, Mound A, Lambert de Rouvroit C, Poumay Y. Modelling atopic dermatitis during the morphogenetic process involved in reconstruction of a human epidermis. Curr Res Transl Med. 2016;64(4):179–83.
Ulzii D, Kido-Nakahara M, Nakahara T, Tsuji G, Furue K, Hashimoto-Hachiya A, et al. Scratching counteracts IL-13 signaling by upregulating the decoy receptor IL-13Ralpha2 in keratinocytes. Int J Mol Sci. 2019;20(13): 3324.
Kasaian MT, Raible D, Marquette K, Cook TA, Zhou S, Tan XY, et al. IL-13 antibodies influence IL-13 clearance in humans by modulating scavenger activity of IL-13Ralpha2. J Immunol. 2011;187(1):561–9.
Wood N, Whitters MJ, Jacobson BA, Witek J, Sypek JP, Kasaian M, et al. Enhanced interleukin (IL)-13 responses in mice lacking IL-13 receptor alpha 2. J Exp Med. 2003;197(6):703–9.
Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008;126(3):332–7.
Popovic B, Breed J, Rees DG, Gardener MJ, Vinall LM, Kemp B, et al. Structural characterisation reveals mechanism of IL-13-neutralising monoclonal antibody tralokinumab as inhibition of binding to IL-13Ralpha1 and IL-13Ralpha2. J Mol Biol. 2017;429(2):208–19.
Wollenberg A, Howell MD, Guttman-Yassky E, Silverberg JI, Kell C, Ranade K, et al. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J Allergy Clin Immunol. 2019;143(1):135–41.
Ultsch M, Bevers J, Nakamura G, Vandlen R, Kelley RF, Wu LC, et al. Structural basis of signaling blockade by anti-IL-13 antibody Lebrikizumab. J Mol Biol. 2013;425(8):1330–9.
Simpson EL, Flohr C, Eichenfield LF, Bieber T, Sofen H, Taieb A, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol. 2018;78(5):863–71.e11.
Dermira Presents Data From Phase 2b Study of Lebrikizumab in Patients With Atopic Dermatitis at Fall Clinical Dermatology Conference
: Business Wire; 2019 https://www.businesswire.com/news/home/20191017005896/en/. Accessed 19 Oct 2019
Dermira Announces Initiation of Phase 3 Program Evaluating Lebrikizumab in Patients with Moderate-to-Severe Atopic Dermatiti: Business Wire; 2019 [October 19, 2019]. https://www.businesswire.com/news/home/20191009005218/en/
Nakashima C, Otsuka A, Kabashima K. Interleukin-31 and interleukin-31 receptor: new therapeutic targets for atopic dermatitis. Exp Dermatol. 2018;27(4):327–31.
Miake S, Tsuji G, Takemura M, Hashimoto-Hachiya A, Vu YH, Furue M, et al. IL-4 Augments IL-31/IL-31 Receptor Alpha Interaction Leading to Enhanced Ccl 17 and Ccl 22 Production in Dendritic Cells: Implications for Atopic Dermatitis. Int J Mol Sci. 2019;20(16): 4053.
Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117(2):411–7.
Silverberg JI, Pinter A, Pulka G, Poulin Y, Bouaziz JD, Wollenberg A, et al. Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J Allergy Clin Immunol. 2019;145(1):173-82.
Ungar B, Garcet S, Gonzalez J, Dhingra N, Correa da Rosa J, Shemer A, et al. An integrated model of atopic dermatitis biomarkers highlights the systemic nature of the disease. J Invest Dermatol. 2017;137(3):603–13.
Lou H, Lu J, Choi EB, Oh MH, Jeong M, Barmettler S, et al. Expression of IL-22 in the skin causes Th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial Th2 cytokines and the GRP pathway. J Immunol. 2017;198(7):2543–55.
Guttman-Yassky E, Brunner PM, Neumann AU, Khattri S, Pavel AB, Malik K, et al. Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: a randomized, double-blind, phase 2a trial. J Am Acad Dermatol. 2018;78(5):872–81.e6.
Suarez-Farinas M, Ungar B, Noda S, Shroff A, Mansouri Y, Fuentes-Duculan J, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136(5):1277–87.
Maeda S, Hayami Y, Naniwa T, Ueda R. The Th17/IL-23 axis and natural immunity in psoriatic arthritis. Int J Rheumatol. 2012;2012:539683.
Leonardi S, Cuppari C, Manti S, Filippelli M, Parisi GF, Borgia F, et al. Serum interleukin 17, interleukin 23, and interleukin 10 values in children with atopic eczema/dermatitis syndrome (AEDS): association with clinical severity and phenotype. Allergy Asthma Proc. 2015;36(1):74–81.
Khattri S, Brunner PM, Garcet S, Finney R, Cohen SR, Oliva M, et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis. Exp Dermatol. 2017;26(1):28–35.
Saeki H, Kabashima K, Tokura Y, Murata Y, Shiraishi A, Tamamura R, et al. Efficacy and safety of ustekinumab in Japanese patients with severe atopic dermatitis: a randomized, double-blind, placebo-controlled, phase II study. Br J Dermatol. 2017;177(2):419–27.
Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155(2):285–95.
Indra AK. Epidermal TSLP: a trigger factor for pathogenesis of atopic dermatitis. Expert Rev Proteomics. 2013;10(4):309–11.
Uysal P, Birtekocak F, Karul AB. The relationship between serum TARC, TSLP and POSTN levels and childhood atopic dermatitis. Clin Lab. 2017;63(7):1071–7.
Simpson EL, Parnes JR, She D, Crouch S, Rees W, Mo M, et al. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: a randomized phase 2a clinical trial. J Am Acad Dermatol. 2019;80(4):1013–21.
Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210(13):2939–50.
Murakami-Satsutani N, Ito T, Nakanishi T, Inagaki N, Tanaka A, Vien PT, et al. IL-33 promotes the induction and maintenance of Th2 immune responses by enhancing the function of OX40 ligand. Allergol Int. 2014;63(3):443–55.
Seltmann J, Roesner LM, von Hesler FW, Wittmann M, Werfel T. IL-33 impacts on the skin barrier by downregulating the expression of filaggrin. J Allergy Clin Immunol. 2015;135(6):1659–61.e4.
Ogg G. Proof-of-Concept Phase-2a Clinical Trial of ANB020 (Anti-IL-33 Antibody) in the Treatment of Moderate-to-Severe Adult Atopic Dermatitis. European Academy of Allergy and Clinical Immunology Congress; United Kingdom: University of Oxford; May 29th 2018.
Anaptsbio reports Etokimab Atlas Phase 2B Clinical Trial In Moderate-To-Severe Atopic Dermatitis Fails to Meet Primary Endpoint [press release]. San Diego: Globe Newswire2019. https://ir.anaptysbio.com/news-releases/news-release-details/anaptysbio-reports-etokimab-atlas-phase-2b-clinical-trial. Accessed 19 Oct 2019.
Vandeghinste N, Klattig J, Jagerschmidt C, Lavazais S, Marsais F, Haas JD, et al. Neutralization of IL-17C reduces skin inflammation in mouse models of psoriasis and atopic dermatitis. J Invest Dermatol. 2018;138(7):1555–633.
Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, Jeet S, Lesch J, et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol. 2011;12(12):1159–66.
MOR106, an Anti-IL-17C mAb, a Potential New Approach for Treatment of Moderate-to-severe Atopic Dermatitis: Phase 1 Study. [press release]. 2018. https://www.morphosys.com/media-investors/media-center/morphosys-and-galapagos-report-first-promising-signs-of-clinical. Accessed 20 Oct 2019.
Morphosys. Morphosys AG: M0R106 Clinical Development in Atopic Dermatitis Stopped. 2019.
Kurschus FC, Moos S. IL-17 for therapy. J Dermatol Sci. 2017;87(3):221–7.
Ilves T, Harvima IT. OX40 ligand and OX40 are increased in atopic dermatitis lesions but do not correlate with clinical severity. J Eur Acad Dermatol Venereol. 2013;27(2):e197–205.
Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9(4):271–85.
Papp KA, Gooderham MJ, Girard G, Raman M, Strout V. Phase I randomized study of KHK4083, an anti-OX40 monoclonal antibody, in patients with mild to moderate plaque psoriasis. J Eur Acad Dermatol Venereol. 2017;31(8):1324–32.
A Phase 1 Study of KHK4083 in subjects with Atopic Dermatitis [press release]. Kyowa Hakko Kirin Co., Ltd.2018. https://ir.kyowakirin.com/en/library/events/main/02/teaserItems1/0/linkList/00/link/181203_02_KHK4083_en.pdf. Accessed 19 Oct 2019.
Guttman-Yassky E, Pavel AB, Zhou L, Estrada YD, Zhang N, Xu H, et al. GBR 830, an anti-OX40, improves skin gene signatures and clinical scores in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144(2):482–93.e7.
Kurzrock R, Hickish T, Wyrwicz L, Saunders M, Wu Q, Stecher M, et al. Interleukin-1 receptor antagonist levels predict favorable outcome after bermekimab, a first-in-class true human interleukin-1alpha antibody, in a phase III randomized study of advanced colorectal cancer. Oncoimmunology. 2019;8(3):1551651.
Bou-Dargham MJ, Khamis ZI, Cognetta AB, Sang QA. The role of interleukin-1 in inflammatory and malignant human skin diseases and the rationale for targeting interleukin-1 alpha. Med Res Rev. 2017;37(1):180–21616.
Dr. Eric Simpson to Present Bermekimab Results in Atopic Dermatitis at 2019 AAD Annual Meeting [press release]. 2019. https://investors.xbiotech.com/news-releases/news-release-details/dr-eric-simpson-present-bermekimab-results-atopic-dermatitis. Accessed 21 Oct 2019.
Milgrom H, Fick RB Jr, Su JQ, Reimann JD, Bush RK, Watrous ML, et al. Treatment of allergic asthma with monoclonal anti-IgE antibody. rhuMAb-E25 Study Group. N Engl J Med. 1999;341(26):1966–73.
Lane JE, Cheyney JM, Lane TN, Kent DE, Cohen DJ. Treatment of recalcitrant atopic dermatitis with omalizumab. J Am Acad Dermatol. 2006;54(1):68–72.
Krathen RA, Hsu S. Failure of omalizumab for treatment of severe adult atopic dermatitis. J Am Acad Dermatol. 2005;53(2):338–40.
Heil PM, Maurer D, Klein B, Hultsch T, Stingl G. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course—a randomized, placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges. 2010;8(12):990–8.
Holm JG, Thomsen SF. Omalizumab for atopic dermatitis: evidence for and against its use. G Ital Dermatol Venereol. 2019;154(4):480–7.
Hotze M, Baurecht H, Rodriguez E, Chapman-Rothe N, Ollert M, Folster-Holst R, et al. Increased efficacy of omalizumab in atopic dermatitis patients with wild-type filaggrin status and higher serum levels of phosphatidylcholines. Allergy. 2014;69(1):132–5.
Chan S, Cornelius V, Chen T, Radulovic S, Wan M, Jahan R, et al. Atopic Dermatitis Anti-IgE Paediatric Trial (ADAPT): the role of anti-IgE in severe paediatric eczema: study protocol for a randomised controlled trial. Trials. 2017;18(1):136 (CQGE031X2201 NCTN).
Haldar P. Patient profiles and clinical utility of mepolizumab in severe eosinophilic asthma. Biologics. 2017;11:81–95.
Oldhoff JM, Darsow U, Werfel T, Katzer K, Wulf A, Laifaoui J, et al. Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy. 2005;60(5):693–6.
Author information
Authors and Affiliations
Contributions
Wenelia Baghoomian performed the literature search and drafted the paper, ChanHo Na and Eric Simpson critically revised the work.
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this review.
Conflict of interest
There are no conflict of interest for Wenelia Baghoomian and ChanHo Na. Dr Simpson reports grants and personal fees from AbbVie, grants and personal fees from Eli Lilly, grants from Galderma, grants from Kyowa Hakko Kirin, grants and personal fees from Leo Pharmaceutical, grants from Merck, grants and personal fees from Pfizer, grants and personal fees from Regeneron, personal fees from Sanofi, personal fees from Dermira, grants from Galderma, grants and personal fees from MedImmune, grants from Novartis, grants from Tioga, grants from Celgene, personal fees from Boehringer-Ingelheim, personal fees from Dermavant, personal fees from Forte Bio, personal fees from Incyte, personal fees from Menlo Therapeutics, personal fees from Ortho Dermatologics, personal fees from Pierre Fabre Dermo Cosmetique, personal fees from Valeant.
Rights and permissions
About this article
Cite this article
Baghoomian, W., Na, C. & Simpson, E.L. New and Emerging Biologics for Atopic Dermatitis. Am J Clin Dermatol 21, 457–465 (2020). https://doi.org/10.1007/s40257-020-00515-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-020-00515-1