Abstract
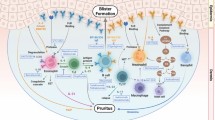
Bullous pemphigoid (BP) is the most common autoimmune subepidermal blistering disease in Western countries, and typically affects the elderly. BP is immunologically characterized by tissue-bound and circulating autoantibodies directed against either the BP antigen 180 (BP180, or BPAG2) or the BP antigen 230 (BP230, or BPAG1e), or even both, which are components of hemidesmosomes involved in the dermal–epidermal cohesion. Risk factors for BP include old age, neurologic diseases (dementia, Parkinson’s disease, cerebrovascular disease), and some particular drugs, including loop diuretics, spironolactone and neuroleptics. The spectrum of clinical presentations is extremely broad. Clinically, BP is an intensely pruritic erythematous eruption with widespread blister formation. In the early stages, or in atypical, non-bullous variants of the disease, only excoriated, eczematous or urticarial lesions (either localized or generalized) are present. The diagnosis of BP relies on immunopathologic findings, especially based on both direct and indirect immunofluorescence microscopy observations, as well as on anti-BP180/BP230 enzyme-linked immunosorbent assays (ELISAs). BP is usually a chronic disease, with spontaneous exacerbations and remissions, which may be accompanied by significant morbidity. In the past decade, potent topical corticosteroids have emerged as an effective and safe first-line treatment for BP, but their long-term feasibility is still controversial. Newer therapeutic agents targeting molecules involved in the inflammatory cascade associated with BP represent future alternatives to classical immunosuppressant drugs for maintenance therapy.

Similar content being viewed by others
References
Stanley JR. Pemphigus and pemphigoid as paradigms of organ-specific, autoantibody-mediated diseases. J Clin Invest. 1989;83:1443–8.
Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381:320–32.
Stanley JR, Tanaka T, Mueller S, et al. Isolation of complementary DNA for bullous pemphigoid antigen by use of patients’ autoantibodies. J Clin Invest. 1988;82:1864–70.
Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol. 1992;99:243–50.
Zillikens D, Rose PA, Balding SD, et al. Tight clustering of extracellular BP180 epitopes recognized by bullous pemphigoid autoantibodies. J Invest Dermatol. 1997;109:573–9.
Thoma-Uszynski S, Uter W, Schwietzke S, et al. Autoreactive T and B cells from bullous pemphigoid (BP) patients recognize epitopes clustered in distinct regions of BP180 and BP230. J Immunol. 2006;176:2015–23.
Di Zenzo G, Thoma-Uszynski S, Fontao L, et al. Multicenter prospective study of the humoral autoimmune response in bullous pemphigoid. Clin Immunol. 2008;128:415–26.
Giudice GJ, Wilske KC, Anhalt GJ, et al. Development of an ELISA to detect anti-BP180 autoantibodies in bullous pemphigoid and herpes gestationis. J Invest Dermatol. 1994;102:878–81.
Zillikens D, Mascaro JM, Rose PA, Liu Z, Ewing SM, Caux F, et al. A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Invest Dermatol. 1997;109:679–83.
Kobayashi M, Amagai M, Kuroda-Kinoshita K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci. 2002;30:224–32.
Di Zenzo G, Grosso F, Terracina M, et al. Characterization of the anti-BP180 autoantibody reactivity profile and epitope mapping in bullous pemphigoid patients. J Invest Dermatol. 2004;122:103–10.
Sakuma-Oyama Y, Powell AM, et al. Evaluation of a BP180-NC16a enzyme-linked immunosorbent assay in the initial diagnosis of bullous pemphigoid. Br J Dermatol. 2004;151:126–31.
Thoma-Uszynski S, Uter W, Schwietzke S, et al. BP230- and BP180-specific auto-antibodies in bullous pemphigoid. J Invest Dermatol. 2004;122:1413–22.
Tsuji-Abe Y, Akiyama M, Yamanaka Y, et al. Correlation of clinical severity and ELISA indices for the NC16A domain of BP180 measured using BP180 ELISA kit in bullous pemphigoid. J Dermatol Sci. 2005;37:145–9.
Di Zenzo G, Thoma-Uszynski S, Calabresi V, et al. Demonstration of epitope-spreading phenomena in bullous pemphigoid: results of a prospective multicenter study. J Invest Dermatol. 2011;131:2271–80.
Charneux J, Lorin J, Vitry F, et al. Usefulness of BP230 and BP180-NC16a enzyme-linked immunosorbent assays in the initial diagnosis of bullous pemphigoid: a retrospective study of 138 patients. Arch Dermatol. 2011;147:286–91.
Gammon WR, Merritt CC, Lewis DM, et al. An in vitro model of immune complex-mediated basement membrane zone separation caused by pemphigoid antibodies, leukocytes, and complement. J Invest Dermatol. 1982;78:285–90.
Sitaru C, Schmidt E, Petermann S, et al. Autoantibodies to bullous pemphigoid antigen 180 induce dermal-epidermal separation in cryosections of human skin. J Invest Dermatol. 2002;118:664–71.
Liu Z, Diaz LA, Troy JL, et al. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest. 1993;92:2480–8.
Nishie W, Sawamura D, Goto M, et al. Humanization of autoantigen. Nat Med. 2007;13:378–83.
Ujiie H, Shibaki A, Nishie W, et al. A novel active mouse model for bullous pemphigoid targeting humanized pathogenic antigen. J Immunol. 2010;184:2166–74.
Bernard P, Vaillant L, Labeille B, et al. Incidence and distribution of subepidermal autoimmune bullous skin diseases in three French regions. Bullous Diseases French Study Group. Arch Dermatol. 1995;131:48–52.
Zillikens D, Wever S, Roth A, et al. Incidence of autoimmune subepidermal blistering dermatoses in a region of central Germany. Arch Dermatol. 2005;131:957–8.
Jung M, Kippes W, Messer G, et al. Increased risk of bullous pemphigoid in male and very old patients: a population-based study on incidence. J Am Acad Dermatol. 1999;41:266–8.
Gudi VS, White MI, Cruikshank N, et al. Annual incidence and mortality of bullous pemphigoid in the Grampian region of north-east Scotland. Br J Dermatol. 2005;153:424–7.
Langan SM, Smeeth L, Hubbard R, et al. Bullous pemphigoid and pemphigus vulgaris incidence and mortality in the UK: population based cohort study. BMJ. 2008;337:a180.
Marazza G, Pham HC, Schärer L, et al. Incidence of bullous pemphigoid and pemphigus in Switzerland: a 2-year prospective study. Br J Dermatol. 2009;161:861–8.
Joly P, Baricault S, Sparsa A, et al. Incidence and mortality of bullous pemphigoid in France. J Invest Dermatol. 2012;132:1998–2004.
Brick K, Weaver C, Lohse CM, et al. Incidence of bullous pemphigoid and mortality of patients with bullous pemphigoid in Olmsted County, Minnesota, 1960 through 2009. J Am Acad Dermatol. 2014;71:92–9.
Försti AK, Jokelainen J, Timonen M, Tasanen K. Increasing incidence of bullous pemphigoid in Northern Finland: a retrospective study database study in Oulu University Hospital. Br J Dermatol. 2014;171:1223–6.
Korman N. Bullous pemphigoid. J Am Acad Dermatol. 1987;16:907–24.
Waisbourd-Zinman O, Ben-Amitai D, Cohen AD, et al. Bullous pemphigoid in infancy: clinical and epidemiologic characteristics. J Am Acad Dermatol. 2008;58:41–8.
Schweiger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
Di Zenzo G, Marazza G, Borradori L. Bullous pemphigoid: physiopathology, clinical features and management. Adv Dermatol. 2007;23:257–88.
Schmidt E, Benoit S, Brocker EB. Bullous pemphigoid with localized umbilical involvement. Acta Derm Venereol. 2009;89:419–20.
Callens A, Vaillant L, Machet MC, et al. Localized atypical pemphigoid on lymphedema following radiotherapy. Acta Derm Venereol. 1993;73:461–4.
Borradori L, Prost C, Woklenstein P, et al. Localized pretibial bullous pemphigoid and pemphigoid nodularis: report of a case. J Am Acad Dermatol. 1992;27:863–7.
Descamps V, Flageul B, Vignon-Pennamen D, et al. Dyshidrosiform pemphigoid: report of three cases. J Am Acad Dermatol. 1992;26:651–2.
Farrell AM, Kirtschig G, Dalziel KL, et al. Childhood vulval pemphigoid: a clinical and immunopathological study of five patients. Br J Dermatol. 1999;140:308–12.
Komine M, Nashiro K, Asahina K, et al. Vesicular pemphigoid. Int J Dermatol. 1992;31:868–70.
Delpuget-Martin N, Bernard P, Bedane C, et al. Pemphigoïde végétante. Ann Dermatol Vénéréol. 1997;124:467–9.
Cliff S, Holden CA. Pemphigoid nodularis: a report of three cases and review of the literature. Br J Dermatol. 1997;136:398–401.
Korman NJ, Woods SG. Erythrodermic bullous pemphigoid is a clinical variant of bullous pemphigoid. Br J Dermatol. 1995;133:967–71.
Cordel N, Courville P, Martel P, et al. Extensive erosive bullous pemphigoid: an atypical and serious clinical variant. Br J Dermatol. 2002;146:537–9.
Cordel N, Chosidow O, Hellot M-F, et al. Neurological disorders in patients with bullous pemphigoid. Dermatology. 2007;215:187–91.
Stinco G, Codutti R, Scarbolo M, Valent F, Patrone P. A retrospective epidemiological study on the association of bullous pemphigoid and neurological diseases. Acta Derm Venereol. 2005;85:136–9.
Chosidow O, Doppler V, Bensimon G, et al. Bullous pemphigoid and amyotrophic lateral sclerosis: a new clue for understanding the bullous disease? Arch Dermatol. 2000;136:521–4.
Jedlickova H, Hlubinka M, Pavlik T, et al. Bullous pemphigoid and internal diseases: a case–control study. Eur J Dermatol. 2010;20:96–101.
Taghipour K, Chi C-C, Vincent A, Groves RW, Venning V, Wojnarowska F. The association of bullous pemphigoid with cerebrovascular disease and dementia: a case–control study. Arch Dermatol. 2010;146:1251–4.
Chen YJ, Wu CY, Lin MW, Chen TJ, Liao KK, Chen YC, et al. Comorbidity profiles among patients with bullous pemphigoid: a nationwide population-based study. Br J Dermatol. 2011;165:593–9.
Langan SM, Groves RW, West J. The relationship between neurological disease and bullous pemphigoid: a population-based case–control study. J Invest Dermatol. 2011;131:631–6.
Bastuji-Garin S, Joly P, Lemordant P, et al. Risk factors for bullous pemphigoid in the elderly. A prospective case–control study. J Invest Dermatol. 2011;131:637–43.
Brick K, Weaver CH, Savica R, et al. A population-based study of the association between bullous pemphigoid and neurologic disorders. J Am Acad Dermatol. 2014;71:1191–7.
Casas-de-la-Asuncion E, Ruano-Ruiz J, Rodriguez-Martin AM, et al. Association between bullous pemphigoid and neurologic diseases: a case–control study in Portuguese patients. Actas Dermosifiliogr. 2014;105:860–5.
Texeira VB, Cabral R, Brites MM, et al. Bullous pemphigoid and comorbidities: a case–control study in Portuguese patients. An Bras Dermatol. 2014;89:274–9.
Chevalier V, Barbe C, Reguiai Z, et al. Impact of neurological diseases on the prognosis of bullous pemphigoid: a retrospective study of 178 patients [in French]. Ann Dermatol Vénéréol. 2016;143:179–86.
Lai YC, Yew YW, Lambert WC. Bullous pemphigoid and its association with neurological diseases: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2016;30:2007–15.
Seppanen A, Suuronen T, Hofmann SC, et al. Distribution of collagen XVII in the human brain. Brain Res. 2007;1158:50–6.
Di Zenzo G, Della Torre R, Zambruno G, Borradori L. Bullous pemphigoid: from the clinic to the bench. Clin Dermatol. 2012;30:3–16.
Vassilieva S. Drug-induced pemphigoid: bullous and cicatricial. Clin Dermatol. 1998;16:379–87.
Stravopoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid. J Eur Acad Dermatol. 2014;28:1133–40.
Bastuji-Garin S, Joly P, Picard-Dahan C, et al. Drugs associated with bullous pemphigoid. A case–control study. Arch Dermatol. 1996;132:272–6.
Lloyd-Lavery A, Chi CC, Wojnarowska F, Taghipour K. The associations between bullous pemphigoid and drug use: a UK case–control study. JAMA Dermatol. 2013;149:58–62.
Bene J, Moulis G, Bennani I, et al. Bullous pemphigoid and dipeptidyl peptidase IV inhibitors: a case-noncase study in the French Pharmacovigilance database. Br J Dermatol. 2016;175(2):296–301.
Ruocco E, Wolf R, Caccavale S, et al. Bullous pemphigoid: associations and management guidelines: facts and controversies. Clin Dermatol. 2013;31:400–12.
Venning VA, Vojnarowska F. The association between and malignant disease: a case–control study. Br J Dermatol. 1990;123:439–45.
Lindelof B, Islam N, Eklund G, et al. Pemphigoid and cancer. Arch Dermatol. 1990;126:66–8.
Ogawa H, Sakuma M, Morioka S, et al. The incidence of internal malignancies in pemphigus and bullous pemphigoid in Japan. J Dermatol Sci. 1995;9:136–41.
Ong E, Goldacre R, Hoang U, et al. Associations between bullous pemphigoid and primary malignant cancers: an English national record linkage study, 1999–2011. Arch Dermatol Res. 2014;306:75–80.
Schulze F, Neumann K, Recke A, et al. Malignancies in pemphigus and pemphigoid diseases. J Invest Dermatol. 2015;135:1445–7.
Kirtschig G, Chow ET, Venning VA, et al. Acquired subepidermal bullous diseases associated with psoriasis: a clinical, immunopathological and immunogenetic study. Br J Dermatol. 1996;135:738–45.
Wilczek A, Sticherling M. Concomitant psoriasis and bullous pemphigoid: coincidence or pathogenic relationship? Int J Dermatol. 2006;45:1353–7.
Le Jan S, Plée J, Vallerand D, et al. Innate immune cell-produced IL-17 sustains inflammation in bullous pemphigoid. J Invest Dermatol. 2014;134:2908–17.
Shipman AR, Cooper S, Wojnarowska F. Autoreactivity to bullous pemphigoid 180: is this the link between subepidermal blistering diseases and oral lichen planus? Clin Exp Dermatol. 2011;36:267–9.
Taylor G, Venning V, Wojnarowska F, et al. Bullous pemphigoid and autoimmunity. J Am Acad Dermatol. 1993;29:181–4.
Chuang TY, Korkij W, Soltani K, et al. Increased frequency of diabetes mellitus in patients with bullous pemphigoid: a case–control study. J Am Acad Dermatol. 1984;11:1099–102.
Oh DD, Zhao CY, Murrell DF. A review of case–control studies on the risk factors for the development of auto-immune blistering diseases. J Eur Acad Dermatol. 2016;30:595–603.
Murrell DF, Daniel BS, Joly P, et al. Definitions and outcome measures for bullous pemphigoid: recommendations by an international panel of experts. J Am Acad Dermatol. 2012;66:479–85.
Levy-Sitbon C, Barbe C, Plee J, et al. Assessment of bullous pemphigoid disease area index during treatment: a prospective study of 30 patients. Dermatology. 2014;229:116–22.
Joly P, Roujeau JC, Benichou J, et al. A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. N Engl J Med. 2002;346:321–7.
Joly P, Roujeau JC, Benichou J, et al. A comparison of two regimens of topical corticosteroids in the treatment of patients with bullous pemphigoid: a multicenter randomized study. J Invest Dermatol. 2009;129:1681–7.
Vaillant L, Bernard P, Joly P, et al. Evaluation of clinical criteria for diagnosis of bullous pemphigoid. Arch Dermatol. 1998;134:1075–80.
Joly P, Courville P, Lok C, et al. Clinical criteria for the diagnosis of bullous pemphigoid: a reevaluation according to immunoblot analysis of patient sera. Dermatology. 2004;208:16–20.
Joly P, Benichou J, Lok C, et al. Prediction of survival for patients with bullous pemphigoid: a prospective study. Arch Dermatol. 2005;141:691–8.
Bernard P, Reguiai Z, Tancrède-Bohin E, et al. Risk factors for relapse in patients with bullous pemphigoid in clinical remission: a multicenter, prospective, cohort study. Arch Dermatol. 2009;145:537–42.
Fichel F, Barbe C, Joly P, et al. Clinical and immunologic factors associated with bullous pemphigoid relapse during the first year of treatment: a multicenter, prospective study. JAMA Dermatol. 2014;150:25–33.
della Torre R, Combescure C, Cortes B, et al. Clinical presentation and diagnostic delay in bullous pemphigoid: a prospective nationwide cohort. Br J Dermatol. 2012;167:1111–7.
Lipsker D, Borradori L. Bullous pemphigoid: what are you ? Urgent need of definitions and diagnostic criteria. Dermatology. 2010;221:131–4.
Schmidt E, della Torre R, Borradori L. Clinical features and practical diagnosis of bullous pemphigoid. Clin Dermatol. 2011;29:427–38.
Gammon WR, Kowalewski C, Chorzelski TP, et al. Direct immunofluorescence studies of sodium chloride-separated skin in the differential diagnosis of bullous pemphigoid and epidermolysis bullosa acquisita. J Am Acad Dermatol. 1990;22:664–70.
Terra JB, Meijer JM, Jonkman MF, Diercks GFH. The n- vs. u-serration is a learnable criterion to differentiate pemphigoid from epidermolysis bullosa acquisita in direct immunofluorescence serration-pattern analysis. Br J Dermatol. 2013;169:100–5.
De Jong MC, Bruins S, Heeres K, et al. Bullous pemphigoid and epidermolysis bullosa acquisita. Differentiation by fluorescence overlay antigen mapping. Arch Dermatol. 1996;132:151–7.
Kelly SE, Wojnarowska F. The use of chemically split tissue in the detection of circulating anti-basement membrane zone antibodies in bullous pemphigoid and cicatricial pemphigoid. Br J Dermatol. 1988;118:31–40.
Gammon WR, Fine JD, Forbes M, et al. Immunofluorescence on split skin for the detection and differentiation of basement membrane zone autoantibodies. J Am Acad Dermatol. 1992;27:79–87.
Sardy M, Kostaki D, Varga R, et al. Comparative study of direct and indirect immunofluorescence and of bullous pemphigoid 180 and 230 enzyme-linked immunosorbent assays for diagnosis of bullous pemphigoid. J Am Acad Dermatol. 2013;69:748–53.
van Beek N, Rentzsch K, Probst C, et al. Serological diagnosis of autoimmune bullous skin diseases: prospective comparison of the BIOCHIP mosaic-based indirect immunofluorescence technique with the conventional multi-step single test strategy. Orphanet J Rare Dis. 2012;7:49.
Labib RS, Anhalt GJ, Patel HP, et al. Molecular heterogeneity of the bullous pemphigoid antigens as detected by immunoblotting. J Immunol. 1986;136:1231–5.
Bernard P, Didierjean L, Denis F, et al. Heterogeneous bullous pemphigoid antibodies : detection and characterization by immunoblotting when absent by indirect immunofluorescence. J Invest Dermatol. 1989;92:171–5.
Bernard P, Aucouturier P, Denis F, et al. Immunoblot analysis of IgG subclasses of circulating antibodies in bullous pemphigoid. Clin Immunol Immunopathol. 1990;54:484–94.
Gohestani R, Kanitakis J, Nicolas JF, et al. Comparative sensitivity of indirect immunofluorescence to immunoblot assay for the detection of circulating antibodies to bullous pemphigoid antigens 1 and 2. Br J Dermatol. 1996;135:74–9.
Barnadas MA, Rubiales MV, González MJ, et al. Enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence testing in a bullous pemphigoid and pemphigoid gestationis. Int J Dermatol. 2008;47:1245–9.
Schmidt E, Obe K, Bröcker EB, Zillikens D. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136:174–8.
Roussel A, Benichou J, Randriamanantany ZA, et al. Enzyme-linked immunosorbent assay for the combination of bullous pemphigoid antigens 1 and 2 in the diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:293–8.
Kromminga A, Sitaru C, Hagel C, et al. Development of an ELISA for the detection of autoantibodies to BP230. Clin Immunol. 2004;111:146–52.
Tampoia M, Lattanzi V, Zucano A, et al. Evaluation of a new ELISA assay for detection of BP230 autoantibodies in bullous pemphigoid. Ann N Y Acad Sci. 2009;1173:15–20.
Le Saché-de Peufeilhoux L, Ingen-Housz-Oro S, Hue S, et al. The value of BP230 enzyme-linked immunosorbent assay in the diagnosis and immunological follow-up of bullous pemphigoid. Dermatology. 2012;224:154–9.
Wieland CN, Confere NI, Gibson LE, et al. Anti-bullous pemphigoid 180 and 230 antibodies in a sample of unaffected subjects. Arch Dermatol. 2010;146:21–5.
van Beek N, Dohse A, Riechert F, et al. Serum autoantibodies against the dermal-epidermal junction in patients with chronic pruritic disorders, elderly individuals and blood donors prospectively recruited. Br J Dermatol. 2014;170:943–7.
Meijer JM, Lamberts A, Pas HH, Jonkman MF. Significantly higher prevalence of circulating bullous pemphigoid-specific IgG autoantibodies in elderly patients with a non-bullous skin disorder. Br J Dermatol. 2015;173:1274–6.
Bernard P, Antonicelli F, Bedane C, et al. Prevalence and clinical significance of anti-Laminin 332 autoantibodies detected by a novel enzyme-linked immunosorbent assay in mucous membrane pemphigoid. JAMA Dermatol. 2013;149:533–40.
Chan LS, Ahmed AR, Anhalt GJ, et al. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol. 2002;138:370–9.
Ghohestani RF, Nicolas JF, Rousselle P, Claudy AL. Diagnostic value of indirect immunofluorescence on sodium chloride-split skin in differential diagnosis of subepidermal autoimmune bullous dermatoses. Arch Dermatol. 1997;133:1102–7.
Schmidt E, Skrobek C, Kromminga A, et al. Cicatricial pemphigoid: IgA and IgG autoantibodies target epitopes on both intra- and extracellular domains of bullous pemphigoid antigen 180. Br J Dermatol. 2001;145:778–83.
Yeh SW, Usman AQ, Ahmed AR. Profile of autoantibody to basement membrane zone proteins in patients with mucous membrane pemphigoid: long-term follow up and influence of therapy. Clin Immunol. 2004;112:268–72.
Oyama N, Setterfield JF, Powell AM, et al. Bullous pemphigoid antigen II (BP180) and its soluble extracellular domains are major autoantigens in mucous membrane pemphigoid: the pathogenic relevance to HLA class II alleles and disease severity. Br J Dermatol. 2006;154:90–8.
Bruch-Gerharz D, Hertl M, Ruzicka T. Mucous membrane pemphigoid: clinical aspects, immunopathological features and therapy. Eur J Dermatol. 2007;17:191–200.
Ludwig R. Clinical presentation, pathogenesis, diagnosis, and treatment of epidermolysis bullosa acquisita. ISRN Dermatol. 2013;2013:812029.
Chen M, Kim GH, Prakash I, Woodley DT. Epidermolysis bullosa acquisita: autoimmunity against to anchoring fibril collagen. Autoimmunity. 2012;45:91–101.
Komorowski L, Müller R, Vorobyev A. Sensitive and specific assays for routine serological diagnosis of epidermolysis bullosa acquisita. J Am Acad Dermatol. 2013;68:e89–95.
Yaoita H, Briggaman RA, Lawley TH. Epidermolysis bullosa acquisita : ultrastructural and immunological studies. J Invest Dermatol. 1981;76:288–92.
Dainichi T, Koga H, Tsuji T, et al. From anti-P200 pemphigoid to anti laminin gamma1 pemphigoid. J Dermatol. 2010;37:231–8.
Groth S, Recke A, Vafia K, et al. Development of a simple ELISA for the detection of autoantibodies in anti-P200 pemphigoid. Br J Dermatol. 2011;164:76–82.
Danichi T, Kurono S, Ohyama B, et al. Anti-laminin gamma-1 pemphigoid. Proc Natl Acad Sci USA. 2009;106:2800–5.
Rieckoff-Cantoni L, Bernard P, Didierjean L, et al. Frequency of bullous pemphigoid-like antibodies as detected by western blotting in pruritic dermatoses. Arch Dermatol. 1992;128:791–4.
Baaker C, Terra JB, Jonkman MF. Bullous pemphigoid as pruritus in the elderly. A common presentation. JAMA Dermatol. 2014;149:950–3.
Kyriakis KP, Paparizos VA, Panteleos DN, Tosca AD. Re-evaluation of the natural course of bullous pemphigoid. A prospective study. Int J Dermatol. 1999;38:909–13.
Bernard P, Bedane C, Bonnetblanc JM. Anti-BP180 autoantibodies as a marker of poor vital prognosis in bullous pemphigoid: a cohort analysis of 94 elderly patients. Br J Dermatol. 1997;136:694–8.
Colbert RL, Allen DM, Eastwood D, et al. Mortality rate of bullous pemphigoid in a US medical center. J Invest Dermatol. 2004;122:1091–5.
Cortés B, Khelifa E, Clivaz L, et al. Mortality rate in bullous pemphigoid: a retrospective monocentric cohort study. Dermatology. 2012;225:320–5.
Roujeau JC, Lok C, Bastuji-Garin S, et al. High risk of death in elderly patients with extensive bullous pemphigoid. Arch Dermatol. 1998;134:465–9.
Rzany B, Partscht K, Jung M, et al. Risk factors for lethal outcome inpatients with bullous pemphigoid: low serum albumin level, high dosage of glucocorticosteroids, and old age. Arch Dermatol. 2002;138:903–8.
Kirtschig G, Middleton P, Bennett C, et al. Interventions for bullous pemphigoid. Cochrane Database Syst Rev. 2010;(10):CD002292.
Burton JL, Harman RM, Peachey RD, et al. A controlled trial of azathioprine in the treatment of pemphigoid [proceedings]. Br J Dermatol. 1978;99:14.
Morel P, Guillaume JC. Treatment of bullous pemphigoid with prednisolone only: 0.75 mg/kg/day versus 1.25 mg/kg/day. A multicenter randomized study. Ann Dermatol Venereol. 1984;111:925–8.
Roujeau JC, Guillaume JC, Morel P, et al. Plasma exchange in bullous pemphigoid. Lancet. 1984;2:486–8.
Guillaume JC, Vaillant L, Bernard P, et al. Controlled trial of azathioprine and plasma exchange in addition to prednisolone in the treatment of bullous pemphigoid. Arch Dermatol. 1993;129:49–53.
Dreno B, Sassolas B, Lacour P, et al. Methylprednisolone versus prednisolone methylsulfobenzoate in pemphigoid: a comparative multicenter study. Ann Dermatol Venereol. 1993;120:518–21.
Fivenson DP, Breneman DL, Rosen GB, et al. Nicotinamide and tetracycline therapy of bullous pemphigoid. Arch Dermatol. 1994;130:753–8.
Liu BG, Li ZY, Di M. Effect of Jingui Shenqi pill combined prednisone on expression of glucocorticoid receptor and its clinical effect in treating bullous pemphigoid patients. Zhonqqo Zhong Xi Ji Jie He Za Zhi. 2006;26:881–4.
Beissert S, Werfel T, Frieling U, et al. A comparison of oral methylprednisolone plus azathioprine or mycophenolate mofetil for the treatment of bullous pemphigoid. Arch Dermatol. 2007;143:1536–42.
Daniel BS, Borradori L, Hall RP, Murrell DF. Evidence-based management of bullous pemphigoid. Dermatol Clin. 2011;29:613–20.
Bernard P, Bedane C, Prost C, et al. Bullous pemphigoid. Guidelines for the diagnosis and treatment. Centres de référence des maladies bulleuses auto-immunes. Société Française de Dermatologie [in French]. Ann Dermatol Venereol. 2011;138:247–51.
Venning VA, Thagipour K, Mohd Mustapa MF, et al. British Association of Dermatologists’ guidelines for the management of bullous pemphigoid. Br J Dermatol. 2012;167:1200–14.
Feliciani C, Joly P, Jonkman MF, et al. Management of bullous pemphigoid: the European Dermatology Forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br J Dermatol. 2015;172:867–77.
Lebrun-Vignes V, Roujeau JC, Bernard P, et al. Prednisone is more effective than prednisolone metasulfobenzoate in the treatment of bullous pemphigoid. Arch Dermatol. 1999;135:89–90.
Terra JB, Potze WJ, Jonkman MF. Whole body application of a potent topical corticosteroid for bullous pemphigoid. J Eur Acad Dermatol Venereol. 2014;28:712–8.
Sobocinski V, Duvert-Lehembre S, Bubenheim M, et al. Assessment of compliance with topical corticosteroids in bullous pemphigoid patients. Br J Dermatol. 2016;174:919–21.
Bézier M, Reguiai Z, Vitry F, et al. Usefulness of thiopurine S-methyltransferase genotypic analysis in patients with autoimmune bullous diseases: a preliminary study. Eur J Dermatol. 2008;18:512–7.
Paul MA, Jorizzo JL, Fleischer AB Jr, White WL. Low-dose methotrexate treatment in elderly patients with bullous pemphigoid. J Am Acad Dermatol. 1994;31:620–5.
Kjellman P, Eriksson H, Berg P. A retrospective analysis of patients with bullous pemphigoid treated with methotrexate. Arch Dermatol. 2008;144:612–6.
Gurcan HM, Ahmed AR. Analysis of current data on the use of methotrexate in the treatment of pemphigus and pemphigoid. Br J Dermatol. 2009;161:723–31.
Du-Thanh A, Merlet S, Maillard H, et al. Combined treatment with low-dose methotrexate and initial short-term superpotent topical steroids in bullous pemphigoid : an open, multicenter, retrospective study. Br J Dermatol. 2011;165:1337–43.
Chave TA, Mortimer NJ, ShaH DS, Hutchinson PE. Chlorambucil as steroid-sparing agent in bullous pemphigoid. Br J Dermatol. 2004;151:1107–8.
Gual A, Iranzo P, Mascaro JM. Treatment of bullous pemphigoid with low-dose cyclophosphamide: a case series of 20 patients. J Eur Acad Dermatol. 2014;28:814–8.
Chalmers JR, Wojnarowska F, Kirtschig G, et al. A randomized controlled trial to compare the safety and effectiveness of doxycycline (200 mg daily) with oral prednisolone (0.5 mk kg−1 daily) for initial treatment of bullous pemphigoid: a protocol for the Bullous Pemphigoid Steroids and Tetracyclines (BLISTER) Trial. Br J Dermatol. 2015;173:227–34.
Venning VA, Millard PR, Wojnarowska F. Dapsone as first line therapy for bullous pemphigoid. Br J Dermatol. 1989;120:83–92.
Bouscarat F, Chosidow O, Picard-Dahan C, et al. Treatment of bullous pemphigoid with dapsone: retrospective study of thirty-six cases. J Am Acad Dermatol. 1996;34:683–4.
Chu J, Bradley M, Marinkovitch MP. Topical tacrolimus is a useful adjunctive therapy for bullous pemphigoid. Arch Dermatol. 2003;139:813–5.
Ko MJ, Chu CY. Topical tacrolimus therapy for localized bullous pemphigoid. Br J Dermatol. 2003;149:1079–81.
Harman KE, Black MM. High-dose intravenous immune globulin for the treatment of autoimmune blistering diseases: an evaluation of its use in 14 cases. Br J Dermatol. 1999;140:865–74.
Ahmed AR. Intravenous immunoglobulin therapy for patients with bullous pemphigoid unresponsive to conventional immunosuppressive treatment. J Am Acad Dermatol. 2001;45:825–35.
Gaitanis G, Alexis I, Pelidou SH, et al. High dose intravenous immunoglobulins in the treatment of adult patients with bullous pemphigoid. Eur J Dermatol. 2012;22:363–9.
Guillot B, Donadio D, Guilhou JJ, et al. Long term plasma exchange therapy in bullous pemphigoid. Acta Derm Venereol. 1986;66:73–5.
Müller PA, Bröcker EB, Klinker E, et al. Adjuvant treatment of recalcitrant bullous pemphigoid with immunoadsorption. Dermatology. 2012;224:224–7.
Schmidt E, Seitz CS, Benoit S, et al. Rituximab in autoimmune bullous diseases: mixed responses and adverse effects. Br J Dermatol. 2007;156:352–6.
Reguiai Z, Tchen T, Perceau G, et al. Efficacy of rituximab in a case of refractory bullous pemphigoid. Ann Dermatol Venereol. 2009;136:431–4.
Kasperkiewicz M, Shimanovitch I, Ludwig R, et al. Rituximab for treatment-refractory pemphigus and pemphigoid. J Am Acad Dermatol. 2011;65:552–8.
Hall RP 3rd, Streilein RD, Hannah DL, McNair PD, Fairley JA, Ronaghy A, et al. Association of serum B-cell activating factor level and proportion of memory and transitional B cells with clinical response after rituximab treatment of bullous pemphigoid patients. J Invest Dermatol. 2013;133:2786–8.
Yamauchi PS, Lowe NJ, Gindi V. Treatment of coexisting bullous pemphigoid and psoriasis with the tumour necrosis factor antagonist etanercept. J Am Acad Dermatol. 2006;54:S121–2.
Ahmed AR, Shetty S, Spigelman S. Treatment of recalcitrant bullous pemphigoid with a novel protocol: a retrospective study with a 6-year follow-up. J Am Acad Dermatol. 2016;74:700–8.
Fairley J, Baum CL, Brandt DS, et al. Pathogenicity of IgE autoimmunity: successful treatment of bullous pemphigoid with omalizumab. J Allergy Clin Immunol. 2009;123:704–5.
Yu KK, Crew AB, Messingham KA, Fairley JA, Woodley DT. Omalizumab therapy for bullous pemphigoid. J Am Acad Dermatol. 2014;71:468–74.
Boussemart L, Jacobelli Batteux F, et al. Auto-immune bullous skin diseases occurring under anti-tumor necrosis factor therapy. Dermatology. 2010;221:201–5.
Plée J, Le Jan S, Giustiniani J, et al. Integrating longitudinal serum IL-17 and IL-23 follow-up, along with autoantibodies, contributes to predict bullous pemphigoid outcome. Sci Rep. 2015;5:18001.
Loget J, Plee J, Antonicelli F, Bernard P. A successful treatment with ustekinumab in a case of relapsing bullous pemphigoid associated with psoriasis. J Eur Acad Dermatol. Epub 12 Oct 2016. doi:10.1111/jdv.14002.
Nakayama C, Fujita Y, Watanabe M, et al. Development of bullous pemphigoid during treatment of psoriatic onycho-pachydermo periostitis with ustekinumab. J Dermatol. 2015;42:996–8.
Cai SC, Lim YL, Li W, et al. Anti-BP180 NC16A titres as an indicator of disease activity and outcome in Asian with bummous pemphigoid. Ann Acad Med Singap. 2015;44:119–26.
Ingen-Housz-Oro S, Plee J, Belmondo T, et al. Positive direct immunofluorescence is of better value than ELISA-BP180 and ELISA-BP230 values for the prediction of relapse after treatment cessation in bullous pemphigoid: a retrospective study of 97 patients. Dermatology. 2015;231:50–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this review.
Conflict of interest
Philippe Bernard and Frank Antonicelli have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Bernard, P., Antonicelli, F. Bullous Pemphigoid: A Review of its Diagnosis, Associations and Treatment. Am J Clin Dermatol 18, 513–528 (2017). https://doi.org/10.1007/s40257-017-0264-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-017-0264-2