Abstract
Introduction
Onychomycosis is a very common fungal infection of the nail apparatus; however, it is very hard to treat, even when the causative agent is identified, and usually requires prolonged systemic antifungal therapy. Until the 1990s, oral treatment options included only griseofulvin and ketoconazole, and the cure rate was very low. New generations of antimycotics, such as fluconazole, itraconazole and terbinafine have improved treatment success.
Methods
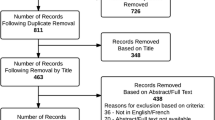
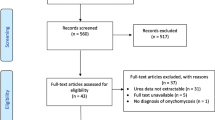
Literature was identified by performing a PubMed Ovid MEDLINE, Ovid EMBASE, EBSCO CINAHL, and Literatura Latino-Americana e do Caribe em Ciências da Saúde (LILACS) search. Prospective and randomized clinical trials were chosen to be included in this review. Forty-six trials were included.
Results
Fluconazole, itraconazole and terbinafine are effective in the treatment of onychomycosis and have a good safety profile. When a dermatophyte is the pathogen, terbinafine produces the best results. For Candida and nondermatophyte infections, the azoles, mainly itraconazole, are the recommended therapy.
Conclusion
In the majority of the studies, terbinafine treatment showed a higher cure ratio than the other drugs for dermatophyte onychomycosis.

Similar content being viewed by others
References
Gupta AK, Jain HC, Lynde CW, et al. Prevalence and epidemiology of onychomycosis in patients visiting physicians offices: a multicenter Canadian survey of 15,000 patients. J Am Acad Dermatol. 2000;43(Pt 1):244–8.
Gupta AK, Jain HC, Lynde CW, et al. Prevalence and epidemiology of unsuspected onychomycosis in patients visiting dermatologists’ offices in Ontario, Canada—a multicenter survey of 2001 patients. Int J Dermatol. 1997;36:783–7.
Gill D, Marks R. A review of the epidemiology of tinea unguium in the community. Australas J Dermatol. 1999;40:6–13.
Scher RK, Coppa LM. Advances in the diagnosis and treatment of onychomycosis. Hosp Med. 1998;34:11–20.
Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415–29.
Heikkilä H, Stubb S. The prevalence of onychomycosis in Finland. Br J Dermatol. 1995;133:699–703.
Dahdah M, Scher R. Onychomycosis—an overview. Mycoses. 2008;46(11–12):496–505.
Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172–3.
Scher RK. Onychomycosis: a significant medical disorder. J Am Acad Dermatol. 1996;35:S2–5.
Finch JJ, Warshaw EM. Toenail onychomycosis: current and future treatment options. Dermatol Ther. 2007;20(1):31–46.
Gupta AK, Sibbald RG, Lynde CW, et al. Onychomycosis in children: prevalence and treatment strategies. J Am Acad Dermatol. 1997;36:395–402.
Reich A, Szepietowski JC. Quality of life in toenail onychomycosis. In: Preedy VR, Watson RR, editors. Handbook of disease burdens and quality of life measures. New York: Springer; 2010. p. 3837–50.
Hay R. Literature review. Onychomycosis. J Eur Acad Dermatol Venereol. 2005;19(Suppl 1):1–7.
Shemer A. Update: medical treatment of onychomycosis. Dermatol Ther. 2012;25(6):582–93.
Singal A, Khanna D. Onychomycosis: diagnosis and management. Indian J Dermatol Venereol Leprol. 2011;77(6):659–72.
Lecha M, Effendy I, de Chauvin MF, et al. Treatment options—development of consensus guidelines. J Eur Acad Dermatol Venereol. 2005;19(suppl 1):25–33.
Grover C, Khurana A. An update on treatment of onychomycosis. Mycoses. 2012;55(6):541–51.
Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11(3):415–29.
Evans EG. Causative pathogens in onychomycosis and the possibility of treatment resistance: a review. J Am Acad Dermatol. 1998;38(Pt 3):S32–6.
Vender RB, Lynde CW, Poulin Y. Prevalence and epidemiology of onychomycosis. J Cutan Med Surg. 2006;10(Suppl 2):S28–33.
Haneke E. Achilles foot-screening project: background, objectives and design. J Eur Acad Dermatol Venereol. 1999;12(Suppl 1):S2–5 (discussion S17).
Burzykowski T, Molenberghs G, Abeck D, Haneke E, Hay R, Katsambas A, et al. High prevalence of foot diseases in Europe: results of the Achilles Project. Mycoses. 2003;46:496–505.
Gupta AK, Fleckman P, Baran R. Ciclopirox nail lacquer topical solution 8% in the treatment of toenail onychomycosis. J Am Acad Dermatol. 2000;43(Suppl 4):S70–80.
Balfour JA, Faulds D. Terbinafine: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in superficial mycoses. Drags. 1992;43:259–84.
Evans EG, Sigurgeirsson B. Double blind, randomised study of continuous terbinafine compared with intermittent itraconazole in treatment of toenail onychomycosis. The LION Study Group. Br J Dermatol. 1999;318:1031–5.
Ryder NS. Terbinafine: mode of action and properties of the squalene epoxidase inhibition. Br J Dermatol. 1992;126(Suppl 39):2–7.
Baudraz-Rosselet F, Rakosi T, Wili PB, et al. Treatment of onychomycosis with terbinafine. Br J Dermatol. 1992;126(Suppl 39):40–6.
Finlay A. Pharmacokinetics of terbinafine in the nail. Br J Dermatol. 1992;126(Suppl. 39):28–32.
Goodfield MJ. Short-duration therapy with terbinafine for dermatophyte onychomycosis: a multicentre trial. Br J Dermatol. 1992;126(Suppl 39):33–5.
Watson A, Marley J, Ellis D, Williams T. Terbinafine in onychomycosis of the toenail: a novel treatment protocol. J Am Acad Dermatol. 1995;33(5 Pt 1):775–9.
Alpsoy E, Yilmaz E, Basaran E. Intermittent therapy with terbinafine for dermatophyte toe-onychomycosis: a new approach. J Dermatol. 1996;23:259–62.
Drake LA, Shear NH, Arlette JP, et al. Oral terbinafine in the treatment of toenail onychomycosis: North American multicenter trial. J Am Acad Dermatol. 1997;37(5 Pt 1):740–5.
Billstein S, Kianifard F, Justice A. Terbinafine vs. placebo for onychomycosis in black patients. Int J Dermatol. 1999;38(5):377–9.
Farkas B, Paul C, Dobozy A, et al. Terbinafine (Lamisil) treatment of toenail onychomycosis in patients with insulin-dependent and non-insulin-dependent diabetes mellitus: a multicentre trial. Br J Dermatol. 2002;146(2):254–60.
Warshaw EM, Fett DD, Bloomfield HE, et al. Pulse versus continuous terbinafine for onychomycosis: a randomized, double-blind, controlled trial. J Am Acad Dermatol. 2005;53(4):578–84.
Takahata Y, Hiruma M, Shiraki Y, et al. Treatment of dermatophyte onychomycosis with three pulses of terbinafine (500 mg day for a week). Mycoses. 2009;52(1):72–6.
Assaf RR, Elewski BE. Intermittent fluconazole dosing in patients with onychomycosis: results of a pilot study. J Am Acad Dermatol. 1996;35(2 Pt 1):216–9.
Scher RK, Breneman D, Rich P, et al. Once-weekly fluconazole (150, 300, or 450 mg) in the treatment of distal subungual onychomycosis of the toenail. J Am Acad Dermatol. 1998;38(6 Pt 2):77–86.
Ling MR, Swinyer LJ, Jarratt MT, et al. Once-weekly fluconazole (450 mg) for 4, 6, or 9 months of treatment for distal subungual onychomycosis of the toenail. J Am Acad Dermatol. 1998;38(6 Pt 2):95–102.
Drake L, Babel D, Stewart DM, et al. Once-weekly fluconazole (150, 300, or 450 mg) in the treatment of distal subungual onychomycosis of the fingernail. J Am Acad Dermatol. 1998;38(6 Pt 2):87–94.
Savin RC, Drake L, Babel D, et al. Pharmacokinetics of three once-weekly dosages of fluconazole (150, 300, or 450 mg) in distal subungual onychomycosis of the fingernail. J Am Acad Dermatol. 1998;38:110–6.
Chen X, Hiruma M, Shiraki Y, et al. Combination therapy of once-weekly fluconazole (100, 150, or 300 mg) with topical application of ketoconazole cream in the treatment of onychomycosis. Jpn J Infect Dis. 2004;57(6):260–3.
Odom R, Daniel CR, Aly R. A double-blind, randomized comparison of itraconazole capsules and placebo in the treatment of onychomycosis of the toenail. J Am Acad Dermatol. 1996;35(1):110–1.
Odom RB, Aly R, Scher RK, et al. A multicenter, placebo-controlled, double-blind study of intermittent therapy with itraconazole for the treatment of onychomycosis of the fingernail. J Am Acad Dermatol. 1997;36(2 Pt 1):231–5.
De Doncker PR, Scher RK, Baran RL, et al. Itraconazole therapy is effective for pedal onychomycosis caused by some nondermatophyte molds and in mixed infection with dermatophytes and molds: a multicenter study with 36 patients. J Am Acad Dermatol. 1997;36(2 Pt 1):173–7.
Chen J, Liao W, Wen H, et al. A comparison among four regimens of itraconazole treatment in onychomycosis. Mycoses. 1999;42(1–2):93–6.
Hiruma M, Matsushita A, Kobayashi M, et al. One week pulse therapy with itraconazole (200 mg day−1) for onychomycosis. Evaluation of treatment results according to patient background. Mycoses. 2001;44(3–4):87–93.
Gupta AK, De Doncker P, Haneke E. Itraconazole pulse therapy for the treatment of Candida onychomycosis. J Eur Acad Dermatol Venereol. 2001;15(2):112–5.
Avner S, Nir N, Baruch K, et al. Two novel itraconazole pulse therapies for onychomycosis: a 2-year follow-up. J Dermatol Treat. 2006;17(2):117–20.
Havu V, Heikkilä H, Kuokkanen K, et al. A double-blind, randomized study to compare the efficacy and safety of terbinafine (Lamisil) with fluconazole (Diflucan) in the treatment of onychomycosis. Br J Dermatol. 2000;142(1):97–102.
Arca E, Taştan HB, Akar A, et al. An open, randomized, comparative study of oral fluconazole, itraconazole and terbinafine therapy in onychomycosis. J Dermatol Treat. 2002;13(1):3–9.
Bräutigam M, Nolting S, Schopf RE, et al. Randomised double blind comparison of terbinafine and itraconazole for treatment of toenail tinea infection. Seventh Lamisil German Onychomycosis Study Group. BMJ. 1995;311(7010):919–22.
Tosti A, Piraccini BM, Stinchi C, et al. Terbinafine therapy with continuous terbinafine treatment and intermittent itraconazole therapy. J Am Acad Dermatol. 1996;34(4):595–600.
De Backer M, De Vroey C, Lesaffre E, Scheys I, De Keyser P. Twelve weeks of continuous oral therapy for toenail onychomycosis caused by dermatophytes: a double-blind comparative trial of terbinafine 250 mg/day versus itraconazole 200 mg/day. J Am Acad Dermatol. 1998;38(5 Pt 3):S57–63.
Sigurgeirsson B, Billstein S, Rantanen T, et al. L.I.ON. Study: efficacy and tolerability of continuous terbinafine (Lamisil) compared to intermittent itraconazole in the treatment of toenail onychomycosis. Lamisil vs. itraconazole in onychomycosis. Br J Dermatol. 1999;141(Suppl 56):5–14.
Bahadir S, Inalöz HS, Alpay K, et al. Continuous terbinafine or pulse itraconazole: a comparative study on onychomycosis. J Eur Acad Dermatol Venereol. 2000;14(5):422–3.
Gupta AK, Konnikov N, Lynde CW. Sequential pulse therapy with itraconazole and terbinafine to treat onychomycosis of the fingernails. J Dermatol Treat. 2000;11:151–4.
Gupta AK, Lynde CW, Konnikov N. Single-blind, randomized, prospective study of sequential itraconazole and terbinafine pulse compared with terbinafine pulse for the treatment of toenail onychomycosis. J Am Acad Dermatol. 2001;44(3):485–91.
Heikkilä H, Stubb S. Long-term results in patients with onychomycosis treated with terbinafine or itraconazole. Br J Dermatol. 2002;146(2):250–3.
Sigurgeirsson B, Olafsson JH, Steinsson JB, et al. Long-term effectiveness of treatment with terbinafine vs itraconazole in onychomycosis: a 5-year blinded prospective follow-up study. Arch Dermatol. 2002;138:353–7.
Warshaw EM, Nelson D, Carver SM, et al. A pilot evaluation of pulse itraconazole vs. terbinafine for treatment of Candida toenail onychomycosis. Int J Dermatol. 2005;44(9):785–8.
Mishra M, Panda P, Tripathy S, et al. An open randomized comparative study of oral itraconazole pulse and terbinafine pulse in the treatment of onychomycosis. Indian J Dermatol Venereol Leprol. 2005;71(4):262–6.
Gupta AK, Gover MD, Lynde CW. Pulse itraconazole vs. continuous terbinafine for the treatment of dermatophyte toenail onychomycosis in patients with diabetes mellitus. J Eur Acad Dermatol Venereol. 2006;20(10):1188–93.
Gupta AK, Lynch LE, Kogan N, et al. The use of an intermittent terbinafine regimen for the treatment of dermatophyte toenail onychomycosis. J Eur Acad Dermatol Venereol. 2009;23(3):256–62.
Piraccini B, Sisti A, Tosti A. Long-term follow-up of toenail onychomycosis caused by dermatophytes after successful treatment with systemic antifungal agents. Dermatology. 2010;62:411–4.
Krishna G, Beresford E, Ma L, et al. Skin concentrations and pharmacokinetics of posaconazole after oral administration. Antimicrob Agents Chemother. 2010;54(5):1807–10.
Elewski B, Pollak R, Ashton S, et al. A randomized, placebo- and active-controlled, parallel-group, multicentre, investigator-blinded study of four treatment regimens of posaconazole in adults with toenail onychomycosis. Br J Dermatol. 2012;166(2):389–98.
Haneke E, Tausch I, Bräutigam M, et al. Short-duration treatment of fingernail dermatophytosis: a randomized, double-blind study with terbinafine and griseofulvin. LAGOS III Study Group. J Am Acad Dermatol. 1995;32(1):72–7.
Faergemann J, Anderson C, Hersle K, et al. Double-blind, parallel-group comparison of terbinafine and griseofulvin in the treatment of toenail onychomycosis. J Am Acad Dermatol. 1995;32(5 Pt 1):750–3.
Hofmann H, Bräutigam M, Weidinger G, et al. Treatment of toenail onychomycosis. A randomized, double-blind study with terbinafine and griseofulvin. LAGOS II Study Group. Arch Dermatol. 1995;131(8):919–22.
Piepponen T, Blomqvist K, Brandt H, et al. Efficacy and safety of itraconazole in the long-term treatment of onychomycosis. J Antimicrob Chemother. 1992;29(2):195–205.
Korting HC, Schäfer-Korting M, Zienicke H, et al. Treatment of tinea unguium with medium and high doses of ultramicrosize griseofulvin compared with that with itraconazole. Antimicrob Agents Chemother. 1993;37(10):2064–8.
Gupta AK, Gregurek-Novak T. Efficacy of itraconazole, terbinafine, fluconazole, griseofulvin and ketoconazole in the treatment of Scopulariopsis brevicaulis causing onychomycosis of the toes. Dermatology. 2001;202:235–8.
Hall M, Monka C, Krupp P, et al. Safety of oral terbinafine: results of a postmarketing surveillance study in 25,884 patients. Arch Dermatol. 1997;133:1213–9.
Gupta AK, Chwetzoff E, Del Rosso F, et al. Hepatic safety of itraconazole. J Cutan Med Surg. 2002;6:210–3.
Garcia-Rodriguez LA, Castellsague AD, Perez-Gutthann S, et al. A cohort study on the risk of acute liver injury among users of ketoconazole and other antifungal drugs. Br J Clin Pharmacol. 1999;48:847–52.
Castellsague J, Garcia-Rodriguez LA, Duque A, et al. Risk of serious skin disorders among users of oral antifungals: a population-based study. BMC Dermatol. 2002;2:14.
Gupta AK, Shear NH. A risk-benefit assessment of the newer oral antifungal agents used to treat onychomycosis. Drug Saf. 2000;22:33–52.
Del Rosso JQ, Gupta AK, Conte ET. How to recognize and manage adverse reactions to oral antifungals. Skin Aging. 1999;42:48–56.
Hall M, Monka C, Krupp R, et al. Safety of oral terbinafine: results of a postmarketing surveillance study in 25, 884 patients. Arch Dermatol. 1997;133:1213–9.
O’Sullivan DP, Needham CA, Bangs A, et al. Postmarketing surveillance of oral terbinafine in the UK: report of a large cohort study. Br J Clin Pharmacol. 1996;42:559–65.
Gupta AK, Sauder DN, Shear NH. Antifungal agents: an overview, part II. J Am Acad Dermatol. 1994;30:911–33.
Elewski BE. Clinical pearl: diagnosis of onychomycosis. J Am Acad Dermatol. 1995;32:500–1.
Epstein WL, Shah VP, Riegelman S. Griseofulvin levels in the stratum corneum: study after oral administration in man. Arch Dermatol. 1972;106:344–8.
Wenig JA. The systemic treatment of onychomycosis. Clin Podiatr Med Surg. 2004;21:579–89.
Meinhof W. Kinetics and spectrum of activity of oral antifungals: the therapeutic implications. J Am Acad Dermatol. 1993;29(1):S39.
Chien RN, Yang LJ, Lin PY, et al. Hepatic injury during ketoconazole therapy in patients with onychomycosis: a controlled cohort study. Hepatology. 1997;25(1):103–7.
Knight T, Shokuma C, Knight J. Ketoconazole-induced fulminant hepatitis necessitating liver transplantation. J Am Acad Dermatol. 1991;25:398–400.
Janssen PAJ, Cauwenbergh G, Symoens J. Hepatic reactions during ketoconazole treatment: a 1-year update. Beerse: Janssen Pharmaceutica; 1983.
Huang PH, Paller AS. Itraconazole pulse therapy for dermatophyte onychomycosis in children. Arch Pediatr Adolesc Med. 2000;154(6):614–8.
Kim TH, Kim BH, Kim YW, et al. Liver cirrhosis developed after ketoconazole-induced acute hepatic injury. J Gastroenterol Hepatol. 2003;18(12):1426–9.
Stricker BH, Block APR, Bronkhorst FB, et al. Ketoconazole associated hepatic injury. J Hepatol. 1986;3:399–406.
Hay RJ. Risk/benefit ratio of modern antifungal therapy: focus on hepatic reactions. J Am Acad Dermatol. 1993;29(1 Suppl):S50–4.
Kuokkanen K, Alava S. Fluconazole in the treatment of onychomycosis caused by dermatophytes. J Dermatol Treat. 1993;3:115–7.
Smith SW, Sealy DP, Schneider E, et al. An evaluation of the safety and efficacy of fluconazole in the treatment of onychomycosis. South Med J. 1995;88:1217–20.
De Doncker P, Decroix J, Pierard GE, et al. Antifungal pulse therapy for onychomycosis: a pharmacokinetic and pharmacodynamic investigation of monthly cycles of 1-week pulse therapy with itraconazole. Arch Dermatol. 1996;132:34–41.
Alcantara R, Garibay JM. Itraconazole therapy in dermatomycoses and vaginal candidiasis: effects and adverse effects profile in a large multi-center study. Adv Ther. 1988;5(6):326–34.
Suhonen R, Neuvonen PJ. The tolerability profile of terbinafine. Rev Contemp Pharmacother. 1997;8:373–86.
Tavakkol A, Fellman S, Kianifard F. Safety and efficacy of oral terbinafine in the treatment of onychomycosis: analysis of the elderly subgroup in Improving Results in Onychomycosis-Concomitant Lamisil and Debridement (IRON-CLAD), an open-label, randomized trial. Am J Geriatr Pharmacother. 2006;4(1):1–13.
Hall M, Monka C, Krupp R, et al. Safety of oral terbinafine: results of a postmarketing surveillance study in 25, 884 patients. Arch Dermatol. 1997;133:1213–9.
O’Sullivan DP, Needham CA, Bangs A, et al. Postmarketing surveillance of oral terbinafine in the UK: report of a large cohort study. Br J Clin Pharmacol. 1996;42:559–65.
Stricker BHC, Van Riemsdijk MM, Stunkenboom MCJM, et al. Taste loss to terbinafine: a case–control study of potential risk factors [abstract]. Pharmacoepidemiol Drug Saf. 1995;4:522.
Chambers WM, Millar A, Jain S, et al. Terbinafine induced hepatic dysfunction. Eur J Gastroenterol Hepatol. 2001;13:1115–8.
Arikian SR, Einarson TR, Kobelt-Nguyen G, et al. A multinational pharmacoeconomic analysis of oral therapies for onychomycosis. The Onychomycosis Study Group. Br J Dermatol. 1994;130(Suppl 43):32–4.
Marchetti A, Piech CT, McGhan WF, et al. Pharmacoeconomic analysis of oral therapies for onychomycosis: a U.S. model. Clin Ther. 1996;18:757–77.
Einarson TR, Arikian SR, Shear NH. Cost-effectiveness analysis for onychomycosis therapy in Canada from a government perspective. Br J Dermatol. 1994;130(Suppl 43):35–44.
Warshaw EM. Evaluating costs for onychomycosis treatments: a practitioner’s perspective. J Am Podiatr Med Assoc. 2006;96:38–52.
Acknowledgments
No sources of funding were used to prepare this article. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Sá, D.C., Lamas, A.P.B. & Tosti, A. Oral Therapy for Onychomycosis: An Evidence-Based Review. Am J Clin Dermatol 15, 17–36 (2014). https://doi.org/10.1007/s40257-013-0056-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-013-0056-2