Abstract
Objective
Vericiguat is a new medication to demonstrate clinical efficacy in heart failure with reduced ejection fraction (HFrEF) after worsening heart failure (WHF) events, but its cost–utility was unknown. We aimed to assess the cost–utility of combining the application of vericiguat with standard treatment in HFrEF patients who had WHF events.
Methods
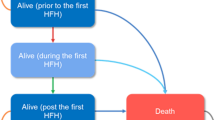
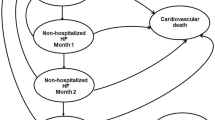
A multistate Markov model was implemented to mimic the economic results of HFrEF patients who had WHF events in China after receiving vericiguat or placebo. An analysis of cost–utility was conducted; most parameters were set according to the published studies and related databases. All the utilities and costs were decreased at a rate of 5% annually. The incremental cost-effectiveness ratios (ICERs) were the primary outcome measure. We also conducted sensitivity analyses.
Results
Over a 20 year lifetime horizon, additional use of vericiguat led to an elevated cost from US$9725.03 to US$20,660.76 at the current vericiguat costs. This was related to increased quality-adjusted life years (QALYs) from 2.50 to 2.66, along with an ICER of US$65,057.24 per QALY, which was over the willingness-to-pay (WTP) threshold of US$36,096.30 per QALY. If the vericiguat costs were discounted at 80%, it contributed to an ICER of US$12,226.77 per QALY. Additional use of vericiguat for patients with plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) of ≤ 5314 pg per ml produced an ICER of US$23,688.46 per QALY. The outcomes of the one-way sensitivity analysis showed the risk of death from cardiovascular disease in both groups was variable with the highest sensitivity. The probabilistic sensitivity analysis showed that 41.6% of the mimicked population receiving vericiguat combined with standard therapy was cost-effective at the WTP threshold of US$36,096.30 per QALY.
Conclusions
From the perspective of Chinese public healthcare system, the combined use of vericiguat and standard treatment in patients with HFrEF following WHF events did not generate advantages in cost–utility in China but was a cost-effective therapeutic strategy for those who with plasma NT-proBNP of ≤ 5314 pg per ml.




Similar content being viewed by others
References
Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure-associated hospitalizations in the United States. J Am Coll Cardiol. 2013;61:1259–67.
Gheorghiade M, Greene SJ, Butler J, Filippatos G, Lam CS, Maggioni AP, et al. Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: the SOCRATES-REDUCED randomized trial. JAMA-J Am Med Assoc. 2015;314:2251–62.
Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391:572–80.
Butler J, Djatche LM, Sawhney B, Chakladar S, Yang L, Brady JE, et al. Clinical and economic burden of chronic heart failure and reduced ejection fraction following a worsening heart failure event. Adv Ther. 2020;37:4015–32.
Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol. 2014;171:368–73.
Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation. 2011;123:2263–73.
Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, et al. Vericiguat in patients with heart failure and reduced ejection fraction. New Engl J Med. 2020;382:1883–93.
Li L, Liu R, Jiang C, Du X, Huffman MD, Lam C, et al. Assessing the evidence-practice gap for heart failure in China: the Heart Failure Registry of Patient Outcomes (HERO) study design and baseline characteristics. Eur J Heart Fail. 2020;22:646–60.
Liu G. The guideline of pharmacoeconomic evaluation of China: 2020. 1ST ed. Peking: China Market Press; 2020. p. 27.
Jiang Y, Zheng R, Sang H. Cost-effectiveness of adding SGLT2 inhibitors to standard treatment for heart failure with reduced ejection fraction patients in China. Front Pharmacol. 2021;12: 733681.
Tang Y, Sang H. Cost-utility analysis of add-on dapagliflozin in heart failure with preserved or mildly reduced ejection fraction. ESC Heart Fail. 2023;10:2524–33.
Tang Y, Sang H. Cost-utility analysis of empagliflozin in heart failure patients with reduced and preserved ejection fraction in China. Front Pharmacol. 2022;13:1030642.
Latif A, Lateef N, Lundgren S, Kapoor V, Ahsan MJ, Aboeata A. Vulnerable phase of acute heart failure and its association with hospital readmissions reduction program. Curr Prob Cardiol. 2022;47: 100904.
DeVore AD, Hammill BG, Sharma PP, QuallsLG, Mentz RJ, Waltman JK, et al. In-hospital worsening heart failure and associations with mortality, readmission, and healthcare utilization. J Am Heart Assoc. 2014;3:e001088.
Desai AS, Claggett BL, Packer M, Zile MR, Rouleau JL, Swedberg K, et al. Influence of sacubitril/valsartan (LCZ696) on 30-day readmission after heart failure hospitalization. J Am Coll Cardiol. 2016;68:241–8.
National Center for Chronic and Noncommunicable Disease Control and Prevention; Chinese Center for Disease Control and Prevention. In: China mortality surveillance dataset 2020. Beijing: China Science and Technology Press; 2021. p. 245–246.
Park SK, Hong SH, Kim H, Kim S, Lee EK. Cost-utility analysis of sacubitril/valsartan use compared with standard care in chronic heart failure patients with reduced ejection fraction in South Korea. Clin Ther. 2019;41:1066–79.
King JB, Shah RU, Bress AP, Nelson RE, Bellows BK. Cost-effectiveness of sacubitril-valsartan combination therapy compared with enalapril for the treatment of heart failure with reduced ejection fraction. JACC-Heart Fail. 2016;4:392–402.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726.
Huang J, Yin H, Zhang M, Ni Q, Xuan J. Understanding the economic burden of heart failure in China: impact on disease management and resource utilization. J Med Econ. 2017;20:549–53.
Qiao JH, Jiang TF, Dai BB, et al. Research on the hospitalization expenses standard of patients with heart failure based on diagnosis related groups. Soft Sci Health. 2022;36:50–4.
.Bank of China, Foreign Exchange Rates, 2022.
Zhu S, Zhang M, Ni Q, Sun Q, Xuan J. Indirect, direct non-medical cost and QOL by New York heart association (NYHA) classification in Chinese heart failure patients. Vaule Health. 2017;20:A268.
Liang L, Bin-Chia WD, Aziz M, Wong R, Sim D, Leong K, et al. Cost-effectiveness of sacubitril/valsartan versus enalapril in patients with heart failure and reduced ejection fraction. J Med Econ. 2018;21:174–81.
National Bureau of statistics of the people’s Republic of China. China health statistics yearbook 2021. Beijing: Peking Union Medical College Press; 2021.
Senni M, Lopez-Sendon J, Cohen-Solal A, Ponikowski P, Nkulikiyinka R, Freitas C, et al. Vericiguat and NT-proBNP in patients with heart failure with reduced ejection fraction: analyses from the VICTORIA trial. ESC Heart Fail. 2022;9:3791–803.
Coats A, Tolppanen H. Drug treatment of heart failure with reduced ejection fraction: defining the role of vericiguat. Drugs. 2021;81:1599–604.
Hulot JS, Trochu JN, Donal E, Galinier M, Logeart D, De Groote P, et al. Vericiguat for the treatment of heart failure: mechanism of action and pharmacological properties compared with other emerging therapeutic options. Expert Opin Pharmacotherapy. 2021;22:1847–55.
Follmann M, Ackerstaff J, Redlich G, Wunder F, Lang D, Kern A, et al. Discovery of the soluble guanylate cyclase stimulator vericiguat (BAY 1021189) for the treatment of chronic heart failure. J Med Chem. 2017;60:5146–61.
Yu X, Hao Y, Zhu Z, Zhang W, Liu B, Ma M, et al. Vericiguat for the treatment of heart failure with reduced ejection fraction following a worsening heart failure event: a cost-effectiveness analysis from the perspective of Chinese healthcare providers. Clin Drug Invest. 2023;43:241–50.
McMurray J, Trueman D, Hancock E, Cowie MR, Briggs A, Taylor M, et al. Cost-effectiveness of sacubitril/valsartan in the treatment of heart failure with reduced ejection fraction. Heart. 2018;104:1006–13.
Alsumali A, Djatche LM, Briggs A, Liu R, Diakite I, Patel D, et al. Cost effectiveness of vericiguat for the treatment of chronic heart failure with reduced ejection fraction following a worsening heart failure event from a US Medicare perspective. Pharmacoeconomics. 2021;39:1343–54.
Butler J, Anstrom KJ, Armstrong PW. Comparing the benefit of novel therapies across clinical trials: insights from the VICTORIA trial. Circulation. 2020;142:717–9.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. New Engl J Med. 2014;371:993–1004.
Colombo G, Casella R, Cazzaniga A, Casiraghi C. Dapagliflozin in patients with heart failure and reduced ejection fraction. Intern Emerg Med. 2020;15:515–7.
Nguyen NV, Lindberg F, Benson L, Ferrannini G, Imbalzano E, Mol P, et al. Eligibility for vericiguat in a real-world heart failure population according to trial, guideline and label criteria: data from the Swedish Heart Failure Registry. Eur J Heart Fail. 2023;25:1418-28
Khan MS, Xu H, Fonarow GC, Lautsch D, Hilkert R, Allen LA, et al. Applicability of vericiguat to patients hospitalized for heart failure in the United States. JACC-Heart Fail. 2023;11:211–23.
Acknowledgements
Thanks to Rui Luo for her writing advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
Penglei Chen, Yixiang Wang, Xin Liu, Jiaqi Yu, and Xuwei Zheng declare that they have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Code availability
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Author contributions
XW Zheng led the study; PL Chen and YX Wang equally designed the study, built the Markov model, analyzed the data, and contributed the manuscript; LX and JQ Yu collected the related data. All authors have read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, P., Wang, Y., Liu, X. et al. Cost–Utility Analysis of Vericiguat in Heart Failure with Reduced Ejection Fraction After Worsening Heart Failure Events in China. Am J Cardiovasc Drugs (2024). https://doi.org/10.1007/s40256-024-00637-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s40256-024-00637-5