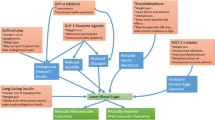
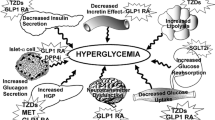
Abstract
With the rising incidence and prevalence rates of type 2 diabetes globally, it is imperative that diabetes prevention strategies are implemented to stem the flow of new cases. Successful interventions include both lifestyle modification and pharmaceutical agents, and large, multicentre, randomised, controlled studies in different populations have identified the benefits of both. However, translating positive trial outcomes to the real world is particularly challenging, as lifestyle interventions require regular reinforcement from healthcare professionals to be maintained. Pharmaceutical therapies may therefore play an adjunctive role in combination with lifestyle to prevent diabetes. Population-based strategies are also necessary to reduce sedentary behaviour and obesity. Well-established glucose-lowering therapies such as metformin, sulphonylureas, thiazolidinediones and insulin and newer agents such as incretin therapies and sodium glucose co-transporter 2 inhibitors have all been investigated in randomised controlled trials for diabetes prevention with varying success. Non-glucose-lowering therapies such as orlistat and renin angiotensin system blockers can prevent diabetes, whereas statins are associated with slightly increased risk. Diabetes prevention strategies should carefully consider the use of these agents according to individual patient circumstances and phenotypic profile.
Similar content being viewed by others
References
International Diabetes F. IDF diabetes atlas; 2015.
Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of type 1 and type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med J Br Diabet Assoc. 2012;29(7):855–62.
Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4–7 yr before clinical diagnosis. Diabetes Care. 1992;15(7):815–9.
Morris DH, Khunti K, Achana F, Srinivasan B, Gray LJ, Davies MJ, et al. Progression rates from HbA1c 6.0-6.4% and other prediabetes definitions to type 2 diabetes: a meta-analysis. Diabetologia. 2013;56(7):1489–93.
Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab. 2005;90(1):493–500.
Stefan N, Fritsche A, Schick F, Haring HU. Phenotypes of prediabetes and stratification of cardiometabolic risk. Lancet Diabetes Endocrinol. 2016;4(9):789–98.
Patel SA, Shivashankar R, Ali MK, Anjana RM, Deepa M, Kapoor D, et al. Is the “South Asian phenotype” unique to South Asians? Comparing cardiometabolic risk factors in the CARRS and NHANES studies. Global Heart. 2016;11(1):89–96.e3.
Stevens JW, Khunti K, Harvey R, Johnson M, Preston L, Woods HB, et al. Preventing the progression to type 2 diabetes mellitus in adults at high risk: a systematic review and network meta-analysis of lifestyle, pharmacological and surgical interventions. Diabetes Res Clin Pract. 2015;107(3):320–31.
Yuen A, Sugeng Y, Weiland TJ, Jelinek GA. Lifestyle and medication interventions for the prevention or delay of type 2 diabetes mellitus in prediabetes: a systematic review of randomised controlled trials. Aust N Z J Public Health. 2010;34(2):172–8.
Merlotti C, Morabito A, Pontiroli AE. Prevention of type 2 diabetes; a systematic review and meta-analysis of different intervention strategies. Diabetes Obes Metab. 2014;16(8):719–27.
Phung OJ, Sood NA, Sill BE, Coleman CI. Oral anti-diabetic drugs for the prevention of type 2 diabetes. Diabet Med J Br Diabet Assoc. 2011;28(8):948–64.
Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ (Clinical research ed). 2007;334(7588):299.
Gillies CL, Lambert PC, Abrams KR, Sutton AJ, Cooper NJ, Hsu RT, et al. Different strategies for screening and prevention of type 2 diabetes in adults: cost effectiveness analysis. BMJ (Clinical research ed). 2008;336(7654):1180–5.
Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2017 executive summary. Endocr Pract. 2017;23(2):207–38.
Pernicova I, Korbonits M. Metformin—mode of action and clinical implications for diabetes and cancer. Nature Rev Endocrinol. 2014;10(3):143–56.
Wang L, Weinshilboum R. Metformin pharmacogenomics: biomarkers to mechanisms. Diabetes. 2014;63(8):2609–10.
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403.
Diabetes Prevention Program Research G. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3(11):866–75.
Diabetes Prevention Program Research G. The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care. 2012;35(4):723–30.
Salpeter SR, Buckley NS, Kahn JA, Salpeter EE. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149–57.e2.
Ramachandran A, Snehalatha C, Shetty AS, Nanditha A. Trends in prevalence of diabetes in Asian countries. World J Diabetes. 2012;3(6):110–7.
Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49(2):289–97.
Weber MB, Ranjani H, Staimez LR, Anjana RM, Ali MK, Narayan KM, et al. The stepwise approach to diabetes prevention: results from the D-CLIP randomized controlled trial. Diabetes Care. 2016;39(10):1760–7.
American Diabetes A. Prevention or delay of type 2 diabetes. Diabetes Care. 2015;38(suppl 1):S31–2.
Lindblad U, Lindberg G, Mansson NO, Ranstam J, Tyrberg M, Jansson S, et al. Can sulphonylurea addition to lifestyle changes help to delay diabetes development in subjects with impaired fasting glucose? The Nepi ANtidiabetes StudY (NANSY). Diabetes Obes Metab. 2011;13(2):185–8.
Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290(4):486–94.
Kahn SE, Lachin JM, Zinman B, Haffner SM, Aftring RP, Paul G, et al. Effects of rosiglitazone, glyburide, and metformin on beta-cell function and insulin sensitivity in ADOPT. Diabetes. 2011;60(5):1552–60.
Kohlroser J, Mathai J, Reichheld J, Banner BF, Bonkovsky HL. Hepatotoxicity due to troglitazone: report of two cases and review of adverse events reported to the United States Food and Drug Administration. Am J Gastroenterol. 2000;95(1):272–6.
Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–71.
Kermode-Scott B. Meta-analysis confirms raised risk of bladder cancer from pioglitazone. BMJ (Clinical research ed). 2012;345:e4541.
Loke YK, Singh S, Furberg CD. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ. 2009;180(1):32–9.
Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374(14):1321–31.
Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes. 2002;51(9):2796–803.
Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, et al. Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes. 2006;55(2):517–22.
Investigators DT, Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet (London, England). 2006;368(9541):1096–105.
Investigators DT, Dagenais GR, Gerstein HC, Holman R, Budaj A, Escalante A, et al. Effects of ramipril and rosiglitazone on cardiovascular and renal outcomes in people with impaired glucose tolerance or impaired fasting glucose: results of the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial. Diabetes Care. 2008;31(5):1007–14.
Hanley AJ, Zinman B, Sheridan P, Yusuf S, Gerstein HC, Diabetes Reduction Assessment With Ramipril and Rosiglitazone Medication (DREAM) Investigators. Effect of rosiglitazone and ramipril on {beta}-cell function in people with impaired glucose tolerance or impaired fasting glucose: the DREAM trial. Diabetes Care. 2010;33(3):608–13.
DeFronzo RA, Abdul-Ghani M. Type 2 diabetes can be prevented with early pharmacological intervention. Diabetes Care. 2011;34(Suppl 2):S202–9.
Group NS, Holman RR, Haffner SM, McMurray JJ, Bethel MA, Holzhauer B, et al. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362(16):1463–76.
Nauck MA, Meier JJ. The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016;4(6):525–36.
Uccellatore A, Genovese S, Dicembrini I, Mannucci E, Ceriello A. Comparison review of short-acting and long-acting glucagon-like peptide-1 receptor agonists. Diabetes Ther Res Treat Educ Diabetes Relat Disord. 2015;6(3):239–56.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22.
Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22.
Kim SH, Abbasi F, Lamendola C, Liu A, Ariel D, Schaaf P, et al. Benefits of liraglutide treatment in overweight and obese older individuals with prediabetes. Diabetes Care. 2013;36(10):3276–82.
le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DC, Van Gaal L, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389(10077):1399–409.
Kalra S. Liraglutide and the rule of halves. Med Matt Diabetes. 2017. https://diabetes.medicinematters.com/editorial-board-comment/12087340
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 2017. doi:10.1056/NEJMoa1611925.
Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1687–93.
Yale JF, Bakris G, Cariou B, Yue D, David-Neto E, Xi L, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15(5):463–73.
Janssen R, Development LLC. CANVAS—Canagliflozin cardiovascular assessment study. 2015.
Davies M, Chatterjee S, Khunti K. The treatment of type 2 diabetes in the presence of renal impairment: what we should know about newer therapies. Clin Pharmacol Adv Appl. 2016;8:61–81.
Investigators OT, Gerstein HC, Bosch J, Dagenais GR, Diaz R, Jung H, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319–28.
Investigators Ot, Gilbert RE, Mann JF, Hanefeld M, Spinas G, Bosch J, et al. Basal insulin glargine and microvascular outcomes in dysglycaemic individuals: results of the Outcome Reduction with an Initial Glargine Intervention (ORIGIN) trial. Diabetologia. 2014;57(7):1325–31.
Bray GA, Fruhbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet (London, England). 2016;387(10031):1947–56.
Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155–61.
Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355(9200):253–9.
Braunwald E, Domanski MJ, Fowler SE, Geller NL, Gersh BJ, Hsia J, et al. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351(20):2058–68.
Dagenais GR, Pogue J, Fox K, Simoons ML, Yusuf S. Angiotensin-converting-enzyme inhibitors in stable vascular disease without left ventricular systolic dysfunction or heart failure: a combined analysis of three trials. Lancet (London, England). 2006;368(9535):581–8.
Navigator study group, McMurray JJ, Holman RR, Haffner SM, Bethel MA, Holzhauer B, et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med.. 2010;362(16):1477–90.
Abuissa H, Jones PG, Marso SP, O’Keefe JH Jr. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta-analysis of randomized clinical trials. J Am Coll Cardiol. 2005;46(5):821–6.
Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet (London, England). 2010;375(9716):735–42.
Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305(24):2556–64.
Sapkota S, Brien JA, Greenfield JR, Aslani P. A systematic review of interventions addressing adherence to anti-diabetic medications in patients with type 2 diabetes—components of interventions. PLoS One. 2015;10(6):e0128581.
Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150(6):1223–34.
El Ouaamari A, Kawamori D, Dirice E, Liew CW, Shadrach JL, Hu J, et al. Liver-derived systemic factors drive beta cell hyperplasia in insulin-resistant states. Cell Rep. 2013;3(2):401–10.
Khunti K, Chatterjee S, Carey M, Daly H, Batista-Ferrer H, Davies MJ. New drug treatments versus structured education programmes for type 2 diabetes: comparing cost-effectiveness. Lancet Diabetes Endocrinol. 2016;4(7):557–9.
Khunti K, Nikolajsen A, Thorsted BL, Andersen M, Davies MJ, Paul SK. Clinical inertia in intensifying therapy among people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab. 2016;18(4):401–9.
Pillinger T, Beck K, Gobjila C, Donocik JG, Jauhar S, Howes OD. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261–9.
Willig AL, Overton ET. Metabolic complications and glucose metabolism in HIV infection: a review of the evidence. Curr HIV/AIDS Rep. 2016;13(5):289–96.
Yudkin JS, Montori VM. The epidemic of pre-diabetes: the medicine and the politics. BMJ (Clinical research ed). 2014;349:g4485.
Collaboration NCDRF. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet (London, England). 2016;387(10027):1513–30.
DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364(12):1104–15.
Investigators DT, Bosch J, Yusuf S, Gerstein HC, Pogue J, Sheridan P, et al. Effect of ramipril on the incidence of diabetes. N Engl J Med. 2006;355(15):1551–62.
Acknowledgments
The authors acknowledge support from the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care—East Midlands (NIHR CLAHRC—EM), the Leicester Clinical Trials Unit, and the NIHR Leicester-Loughborough Diet, Lifestyle and Physical Activity Biomedical Research Centre, which is a partnership between the University Hospitals of Leicester NHS Trust, Loughborough University and the University of Leicester.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Dr Chatterjee has received speaker fees, educational funding, or both from Janssen, Eli Lilly, Novo Nordisk, Astra Zeneca, and Boehringer Ingelheim, and grants in support of investigator-initiated trials from Boehringer Ingelheim and Janssen. Professor Davies reports personal fees from Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, AstraZeneca, Janssen, Mitsubishi Tanabe Pharma Corporation, and Takeda Pharmaceuticals International Inc. and grants from Novo Nordisk, Sanofi-Aventis, Lilly, Boehringer Ingelheim and Janssen. Professor Khunti has acted as a consultant and speaker for Astra Zeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Janssen, and Boehringer Ingelheim. He has received grants in support of investigator and investigator-initiated trials from Astra Zeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Boehringer Ingelheim, Merck Sharp & Dohme, and Roche. Professor Khunti has also served on advisory boards for Astra Zeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Janssen, and Boehringer Ingelheim.
Rights and permissions
About this article
Cite this article
Chatterjee, S., Davies, M. & Khunti, K. Pharmaceutical Interventions for Diabetes Prevention in Patients at Risk. Am J Cardiovasc Drugs 18, 13–24 (2018). https://doi.org/10.1007/s40256-017-0239-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-017-0239-y