Abstract
Background
The extent of P2Y12 inhibition during coronary intervention is an important determinant of ischemic complications. The currently available oral P2Y12 inhibitors are limited by a relatively slow onset of action and variable on-treatment response.
Objective
Our objective was to determine the pharmacodynamic (PD) dose–antiplatelet response relationship and the pharmacokinetics of MDCO-157, an intravenous formulation of clopidogrel complexed with sulphobutylether betacyclodextrin, and to identify the dose level of MDCO-157 that matches the PD effect of oral clopidogrel 300 mg.
Methodology
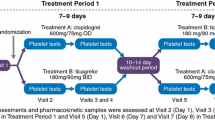
A randomized open-label crossover study was performed in 33 healthy adult volunteers to determine the pharmacokinetic (clopidogrel and clopidogrel H4 thiol active metabolite) and the PD (vasodilator-stimulated phosphoprotein [VASP]) effects of MDCO-157 at doses of 75, 150, and 300 mg and of oral clopidogrel 300 mg.
Results
Data are presented as %, mean (standard deviation). The maximum effect of P2Y12 receptor inhibition assessed by flow cytometry using VASP was 70.42 (6.7), 69.45 (7.1), and 65.58 (12.6) for intravenous MDCO-157 at doses of 75, 150, and 300 mg, respectively, compared with 56.6 (17.5) with oral clopidogrel 300 mg administration (p < 0.0001). Intravenous administration of MDCO-157 led to a stepwise increase in plasma exposure of clopidogrel, higher than with administration of an oral dose of 300 mg (p < 0.0001). Plasma exposure of H4-thiol also increased with intravenous dose (3.6 ± 2.6, 6.9 ± 4.6, and 12.4 ± 9.1 h·ng/ml for intravenous 75, 150, and 300 mg, respectively) but was lower than with oral administration of a 300-mg dose (34.0 ± 16.0 h.ng/ml; pairwise p < 0.0001).
Conclusions
MDCO-157, an intravenous formulation of clopidogrel complexed with sulphobutylether betacyclodextrin, did not show significant platelet inhibition when administered at doses up to 300 mg. Higher doses with longer infusion may be needed to reach a sufficient threshold of active metabolite generation.
Trial Registration: ClinicalTrials.gov identifier: NCT01860105.







Similar content being viewed by others
References
Savi P, Labouret C, Delesque N, Guette F, Lupker J, Herbert JM. P2Y(12), a new platelet ADP receptor, target of clopidogrel. Biochem Biophys Res Commun. 2001;283:379–83.
Savi P, Pereillo JM, Uzabiaga MF, et al. Identification and biological activity of the active metabolite of clopidogrel. Thromb Haemost. 2000;84:891–6.
Collet J-P, Hulot J-S, Anzaha G, et al. High doses of clopidogrel to overcome genetic resistance: the randomized crossover CLOVIS-2 (Clopidogrel and Response Variability Investigation Study 2). JACC Cardiovasc Interv. 2011;4:392–402.
Bellemain-Appaix A, O’Connor SA, Silvain J, et al. Association of clopidogrel pretreatment with mortality, cardiovascular events, and major bleeding among patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. JAMA. 2012;308:2507–16.
Bellemain-Appaix A, Brieger D, Beygui F, et al. New P2Y12 inhibitors versus clopidogrel in percutaneous coronary intervention: a meta-analysis. J Am Coll Cardiol. 2010;56(19):1542–51.
Montalescot G, Wiviott SD, Braunwald E, et al. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet. 2009;373(9665):723–31.
Steg PG, James S, Harrington RA, et al. Ticagrelor versus clopidogrel in patients with st-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: a platelet inhibition and patient outcomes (PLATO) trial subgroup analysis. Circulation. 2010;122(21):2131–41.
Montalescot G, van’t Hof AW, Lapostolle F, et al. Prehospital ticagrelor in ST-segment elevation myocardial infarction. N Engl J Med. 2014;371:1016–27.
Alexopoulos D, Xanthopoulou I, Gkizas V, et al. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. 2012;5:797–804.
Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol. 2015;12:30–47.
Steg PG, Bhatt DL, Hamm CW, et al. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: a pooled analysis of patient-level data. Lancet. 2013;382:1981–92.
European Medicines Agency. http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/003773/WC500180855.pdf. 2015.
Anon. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm452172.htm.
Bhatt DL, Stone GW, Mahaffey KW, et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368:1303–13.
Rollini F, Franchi F, Tello-Montoliu A, et al. Pharmacodynamic effects of cangrelor on platelet P2Y12 receptor-mediated signaling in prasugrel-treated patients. JACC Cardiovasc Interv. 2014;7:426–34.
Angiolillo DJ, Schneider DJ, Bhatt DL, et al. Pharmacodynamic effects of cangrelor and clopidogrel: the platelet function substudy from the cangrelor versus standard therapy to achieve optimal management of platelet inhibition (CHAMPION) trials. J Thromb Thrombolysis. 2012;34:44–55.
Schneider DJ, Agarwal Z, Seecheran N, Keating FK, Gogo P. Pharmacodynamic effects during the transition between cangrelor and ticagrelor. JACC Cardiovasc Interv. 2014;7:435–42.
Schneider DJ, Seecheran N, Raza SS, Keating FK, Gogo P. Pharmacodynamic effects during the transition between cangrelor and prasugrel. Coron Artery Dis. 2015;26:42–8.
Cushing DJ, Souney PF, Cooper WD, et al. Pharmacokinetics and platelet aggregation inhibitory effects of a novel intravenous formulation of clopidogrel in humans: effects of intravenous clopidogrel. Clin Exp Pharmacol Physiol. 2012;39:3–8.
Williams EJ. Experimental designs balanced for the estimation of residual effects of treatments. Aust J Sci Res 1949;2(3):149–168
Montalescot G, Sideris G, Meuleman C, et al. A randomized comparison of high clopidogrel loading doses in patients with non-ST-segment elevation acute coronary syndromes: the ALBION (Assessment of the Best Loading Dose of Clopidogrel to Blunt Platelet Activation, Inflammation and Ongoing Necrosis) trial. J Am Coll Cardiol. 2006;48:931–8.
Collet JP, Silvain J, Landivier A, et al. Dose-effect of clopidogrel re-loading in patients already on 75 mg maintenance dose: the RELOAD study. Circulation. 2008;118:1225–33.
Simon N, Finzi J, Cayla G, Montalescot G, Collet JP, Hulot JS. Omeprazole, pantoprazole, and CYP2C19 effects on clopidogrel pharmacokinetic-pharmacodynamic relationships in stable coronary artery disease patients. Eur J Clin Pharmacol 2015;71(9):1059–66
Pena A, Collet JP, Hulot JS, et al. Can we override clopidogrel resistance? Circulation. 2009;119(21):2854–8.
Hulot JS, Collet JP, Cayla G, et al. CYP2C19 but not PON1 genetic variants influence clopidogrel pharmacokinetics, pharmacodynamics and clinical efficacy in post-myocardial infarction patients. Circ Cardiovasc Interv. 2011;4:422–8.
Frelinger AL 3rd, Bhatt DL, Lee RD, et al. Clopidogrel pharmacokinetics and pharmacodynamics vary widely despite exclusion or control of polymorphisms (CYP2C19, ABCB1, PON1), noncompliance, diet, smoking, co-medications (including proton pump inhibitors), and pre-existent variability in platelet function. J Am Coll Cardiol. 2013;61:872–9.
Windecker S, Alfonso F, Collet J-P, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the ESC/EACTS Developed with the special contribution of the EAPCI. Eur Heart J. 2014;35:2541–619.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Montalescot reports receiving consulting fees from Acuitude, Amgen, AstraZeneca, Bayer, Berlin Chimie AG, Boehringer Ingelheim, Bristol-Myers-Squibb, Brigham Women’s Hospital, Cardiovascular Research Foundation, CME resources, Conway, Daiichi-Sankyo, Eli-Lilly, Europa, Evidera, GLG, Hopitaux Universitaires Genève, Lead-Up, McKinsey & Company, Medcon International, Menarini, Medtronic, MSD, Pfizer, Sanofi-Aventis, Stentys, The Medicines Company, TIMI Study Group, Universität Basel, WebMD, Williams & Connolly, and Zoll Medical; and grant support from ADIR, Amgen, AstraZeneca, Bristol-Myers-Squibb, Celladon, Daiichi-Sankyo, Eli-Lilly, Fédération Française de Cardiologie, Gilead, ICAN, Janssen-Cilag, Pfizer, Recor, Sanofi-Aventis, Stentys. Dr. Silvain reports receiving research grants to the institution from Boehringer-Ingelheim, Daiichi-Sankyo, Eli Lilly, BRAHMS, and Sanofi-Aventis, Fédération Française de Cardiologie and Société Française de Cardiologie, INSERM; consultant fees from Daiichi-Sankyo, Eli Lilly, AstraZeneca, and The Medicines Company; and lecture fees from AstraZeneca, Cordis, Daiichi-Sankyo, Eli Lilly, Iroko Cardio, and STENTYS. Jayne Prats is an employee of The Medicines Company; Ming-yi Hu and Kan He were employees of The Medicines Company. Dr. Collet has received research grants from Bristol-Myers Squibb, Sanofi-Aventis, Eli Lilly, Guerbet Medical, Medtronic, Boston Scientific, Cordis, Stago, Centocor, Fondation de France, INSERM, Fédération Française de Cardiologie, and Société Française de Cardiologie; consulting fees from Sanofi-Aventis, Eli Lilly, and Bristol-Myers Squibb; and lecture fees from Bristol-Myers Squibb, Sanofi-Aventis, and Eli Lilly. Dr. Hulot discloses the following relationships: Data Monitoring Committees: Assistance Publique Hôpitaux de Paris; Honoraria: American College of Cardiology Foundation (Lecturer), Eli Lilly (Steering Committee), Servier (Lecturer); Consulting fees: Daiichi Sankyo (consultant), Imaxio (consultant); Research Grants: Bayer Pharma, Celladon, Cellectis Stem Cells, French Ministry of Health (AOM09191), Institute for Cardiometabolism and Nutrition Foundation, NIH/NHLBI (R01 HL113497), NIH/NGRI (1U01HG006380). The other authors declare no conflicts of interest.
Additional information
N. Nicolas: Deceased.
Rights and permissions
About this article
Cite this article
Collet, JP., Funck-Brentano, C., Prats, J. et al. Intravenous Clopidogrel (MDCO-157) Compared with Oral Clopidogrel: The Randomized Cross-Over AMPHORE Study. Am J Cardiovasc Drugs 16, 43–53 (2016). https://doi.org/10.1007/s40256-015-0145-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-015-0145-0