Abstract
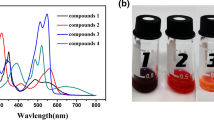
Due to their unique physicochemical properties, the anion radical and dianion of perylene diimide derivatives(PDIs) recently attracted significant attention for organic semiconductors. However, the impact of packing structure and the radical content for carrier transport in the solid state still need to be determined. Bringing the electron-withdrawing groups is an effective strategy for enabling π−π stacking distance. Here, bay-tetrachloro-substituted PDI(B-4Cl-PDI) anion radical and dianion films were fabricated quantitatively doped with N2H4·H2O. The radical contents were quantitatively calculated by absorption spectra in different doping ratios. The X-ray powder diffraction patterns showed that the anion radical presented a crystalline structure, and dianion aggregates exhibited an amorphous structure. With precise manipulation of the radical content, the anion radical aggregates and dianion aggregates showed the maximum electrical conductivity value of 0.024 and 0.0018 S/cm, respectively. The experiment results show that doping level and aggregate structure play a crucial role in electronic transport properties.

Similar content being viewed by others
References
Jones B. A., Facchetti A., Wasielewski M. R., Marks T. J., J. Am. Chem. Soc., 2007, 129, 15259
Schmidt R., Oh J. H., Sun Y.-S., Deppisch M., Krause A.-M., Radacki K., Braunschweig H., Konemann M., Erk P., Bao Z., Wurthner F., J. Am. Chem. Soc., 2009, 131, 6215
Wang H., Yuan S., Ma D., Huang X., Meng F., Zhang X., Adv. Energy Mater., 2014, 4, 1301651
Warczak M., Gryszel M., Jakesova M., Derek V., Glowacki E. D., Chem. Commun., 2018, 54, 1960
Kozma E., Mróz W., Villafiorita-Monteleone F., Galeotti F., Andicsová-Eckstein A., Catellani M., Botta C., RSC Adv., 2016, 6, 61175
Zhang W., Zhong S., Nian L., Chen Y., Xie Z., Liu L., Hanif M., Chen W., Ma Y., RSC Adv., 2015, 5, 39973
Wang Z., Zheng N., Zhang W., Yan H., Xie Z., Ma Y., Huang F., Cao Y., Adv. Energy Mater., 2017, 7, 1700232
Russ B., Robb M. J., Brunetti F. G., Miller P. L., Perry E. E., Patel S. N., Ho V., Chang W. B., Urban J. J., Chabinyc M. L., Hawker C. J., Segalman R. A., Adv. Mater., 2014, 26, 3473
Jiang Q., Zhang J., Mao Z., Yao Y., Zhao D., Jia Y., Hu D., Ma Y., Adv. Mater., 2022, 34, 2108103
Chen Z. X., Li Y., Huang F., Chem, 2021, 7, 288
Rostro L., Baradwaj A. G., Boudouris B. W., ACS Appl. Mater. Interfaces, 2013, 5, 9896
Rostro L., Wong S. H., Boudouris B. W., Macromolecules, 2014, 47, 3713
Joo Y., Agarkar V., Sung S. H., Savoie B. M., Boudouris B. W., Science, 2018, 359, 1391
Kwon T., Koo J. Y., Choi H. C., Angew. Chem. Int. Ed., 2020, 59, 16436
Zhao D., Jiang Q., Jia Y., Zhou J., Zheng N., Hu D., Ma Y., Mater. Today Energy, 2021, 21, 100710
Chen T. A., Rieke R. D., Synth. Met., 1993, 60, 175
Ajayaghosh A., Chem. Soc. Rev., 2003, 32, 181
Tam T. L. D., Ng C. K., Lim S. L., Yildirim E., Ko J., Leong W. L., Yang S., Xu J., Chem. Mater., 2019, 31, 8543
Huang L., Eedugurala N., Benasco A., Zhang S., Mayer K. S., Adams D. J., Fowler B., Lockart M. M., Saghayezhian M., Tahir H., King E. R., Morgan S., Bowman M. K., Gu X., Azoulay J. D., Adv. Funct. Mater., 2020, 1909805
Yuan D., Guo Y., Zeng Y., Fan Q., Wang J., Yi Y., Zhu X., Angew. Chem. Int. Ed., 2019, 58, 4958
Yang K., Zhang X., Harbuzaru A., Wang L., Wang Y., Koh C., Guo H., Shi Y., Chen J., Sun H., Feng K., Delgado R. M. C., Woo H. Y., Ortiz R. P., Guo X., J. Am. Chem. Soc., 2020, 142, 4329
Lutkenhaus J., Science, 2018, 359, 1334
Cardona C. M., Li W., Kaifer A. E., Stockdale D., Bazan G. C., Adv. Mater., 2011, 23, 2367
Sworakowski J., Lipiński J., Janus K., Org. Electron., 2016, 33, 300
Renner R., Stolte M., Heitmüller J., Brixner T., Lambert C., Würthner F., Mater. Horiz., 2022, 9, 350
Seifert S., Schmidt D., Wurthner F., Chem. Sci., 2015, 6, 1663
Dyson F. J., Phys. Rev., 1955, 98, 349
Guy S. C., Edmonds R. N., Edwards P. P., J. Chem. Soc., Faraday Trans. 2, 1985, 81, 937
Acknowledgements
This work was supported by the National Key R&D Program of China (No.2020YFA0714604), the National Natural Science Foundation of China (Nos.U20A6002, 91833304, 51521002 22005107, 52203221), the Basic and Applied Basic Research Major Program of Guangdong Province, China(No. 2019B030302007), the Research and Development Funds for Science and Technology Program of Guangzhou, China(No.202007020004), the Natural Science Foundation of Guangdong Province, China(Nos.2019B121205002, 2022A1515010063), the Fund of the Guangdong Provincial Key Laboratory of Luminescence from Molecular Aggregates, China(No.2019B030301003), and the Funding by Science and Technology Projects in Guangzhou, China (Nos.202102020401, 202102020561).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Conflicts of Interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Jia, Y., Jiang, Q., Wang, B. et al. Electronic Characteristics of Perylene Diimide Anion Radical and Dianion Films by Quantitative Doping. Chem. Res. Chin. Univ. 39, 187–191 (2023). https://doi.org/10.1007/s40242-023-2350-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-023-2350-8