Abstract
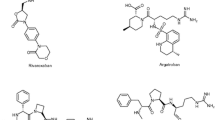
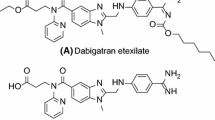
Some 1,2,5-trisubstituted benzimidazole fluorinated derivatives were designed and screened by molecular docking. Five compounds which obtained high scores were selected to synthesize. All the target products were cha-racterized by 1H NMR, 13C NMR and high resolution mass spectra(HRMS) and preliminarily screened for inhibitory activity against thrombin, among which three compounds(5a, 5c and 5e) were evaluated in vitro. The results showed that compounds 5a, 5c and 5e exhibited better anticoagulant activity than argatroban. Docking simulations demon-strated that these compounds may act as candidates for further studies on thrombin inhibitors.
Similar content being viewed by others
References
Dobesh P. P., Fanikos J., Drugs 2014, 74(17), 1
Cohen A. T., Giancarlo A., Anderson F. A., Arcelus J. I., Bergqvist D., Brecht J. G., Greer I. A., Heit J. A., Hutchinson J. L., Kakkar A. K., Mottier D., Oger E., Samama M. M., Spannagl M., Thromb. Haemostasis 2007, 98(4), 756
Perez A., Eraso L. H., Merli G. J., Int. J. Clin. Pract., 2013, 67(2), 139
Coughlin M. A., Bartlett R. H., Asaio J., 2015, 61(6), 652
Robertson L., Kesteven P., Cochrane Db. Syst. Rev. 2015, 12(1), 1
Finazzi G., Ageno W., Intern. Emerg. Med. 2016, 11(2), 167
Schwienhorst A., Cell. Mol. Life Sci. 2006, 63(23), 2773
William B., Hillegass M. M., Catheter. Cardio. Inte., 2016, 87(3), 401
Lee C. J., Ansell J. E., Brit. J. Clin. Pharmaco., 2011, 72(4), 581
Alban S., Curr. Pharm. Design 2008, 14(12), 1152
Kathiresan S., Jin S., Jang I. K., J. Thromb. Thrombolys., 2002, 13(1), 41
Elmasry A. H., Fahmy H. H., Abdelwahed S. H. A., Molecules, 2014, 5(12), 1429
Masashi M., César P. B., Pavel A., Org. Lett. 2011, 13(18), 4882
Sawant R., Kawade D., Acta Pharmaceut. 2011, 61(3), 353
Nile S. H., Brajesh K., Park S. W., Chem. Biol. Drug Des., 2013, 82(3), 290
Gupta R. P., Larroquette C. A., Agrawal K. C., J. Med. Chem., 1982, 25(11), 1342
Hauel N. H., Nar H., Priepke H., Ries U., Stassen J. M., Wienen W., J. Med. Chem. 2002, 45(9), 1757
Hyohdoh I., Furuichi N., Aoki T., Itezono Y., Shirai H., Ozawa S., Watanabe F., Matsushita M., Sakaitani M., Ho P. S., Takanashi K., Harada N., Tomii Y., Yoshinari K., Ori K., Tabo M., Aoki Y., Shimma N., Iikura H., ACS Med. Chem. Lett. 2013, 4(11), 1059
Jiang W., María S. R., JoséL. A., Carlos D. P., Alexander E. S., Santos F., Soloshonok V. A., Liu H., Chem. Rev. 2014, 114(4), 2432
Gillis E. P., Eastman K. J., Hill M. D., Donnelly D. J., Meanwell N. A., J. Med. Chem., 2015, 58(21), 8315
Rowley M., Hallett D. J., Goodacre S., Moyes C., Crawforth J., Sparey T. J., Patel S., Marwood R., Patel S., Thomas S., Hitzel L., Connor C. O., Szeto N., Castro J. L., Hutson P. H., Macleod A. M., J. Med. Chem., 2001, 44(10), 1603
Kirk K. L., J. Fluorine Chem., 2006, 127(8), 1013
Mohamed N. A., El-Serwy W. S., El-Karim S. S. A., Awad G. E., Elseginy S. A., Res. Chem. Intermediat., 2016, 42(2), 1363
Nayak P. S., Narayana B., Sarojini B. K., Fernades J., Bharath B. R., Madhu L. N., Med. Chem. Res., 2015, 24(12), 4191
Li C. L., Dong M. H., Ren Y. J., Li L. H., RSC Adv., 2015, 5(30), 23737
Pilli R. A., Rodrigues M. T., J. Braz. Chem. Soc., 2009, 20(8), 1434
Ren W. X., Ren Y. J., Dong M. H., Gao Y. H., Helv. Chim. Acta, 2016, 4(99), 325
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the “Industry-university-research Institutions” Collaborative Innovation Fund of Shanghai Institute of Technology, China(No.10120K158014-XTCX2015-14).
Rights and permissions
About this article
Cite this article
Yang, H., Ren, Y., Gao, X. et al. Synthesis and anticoagulant bioactivity evaluation of 1,2,5-trisubstituted benzimidazole fluorinated derivatives. Chem. Res. Chin. Univ. 32, 973–978 (2016). https://doi.org/10.1007/s40242-016-6205-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-016-6205-4