Abstract
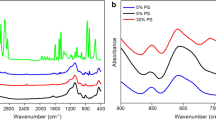
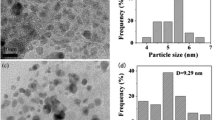
Salicylhydroxamic acid(SHA) was covalently grafted onto chloromethylated crosslinked polystyrene spheres(CMCPS) by the Friedel-Crafts alkylation reaction. The amount of SHA on CPS was found to be mainly dependent on the amount of Lewis acid(SnCl4) used and the reaction temperature. Under optimized conditions, the amount of SHA attached to CPS could reach up to 0.43 g/g CPS. Transition metal ions[Co(II), Cu(II), Fe(III) or Mn(II)] were then introduced into the resulting SHA-functionalized microspheres(SHA/CPS) through SHA-metal ion chelation. The obtained microspheres MSHA/CPS were explored as biomimetic catalysts for the aerobic oxidation of ethylbenzene(EB) to ethylbenzene hydroperoxide(EBHP). Among the four supported metal catalysts, FeSHA/CPS showed the highest catalytic activity and good reusability, indicating its great potential as an effective heterogeneous catalyst for the aerobic oxidation of hydrocarbons under mild conditions.
Similar content being viewed by others
References
Nam N. H., Huong T. L., Dung D. T. M., Dung P. T. P., Oanh D. T. K., Park S. H., Kim K., Han B. W., Yun J., Kang J. S., Kim Y., Han S. B., J. Enzym. Inhib. Med. Chem., 2014, 29(5), 611
Qin Y., Zhao X. J., Fang Y., Int. J. Gynecol. Cancer, 2014, 24(8), 1373
Xu J., Franklin S. J., Whisenhun D. W. J., Raymond K. N., J. Am. Chem. Soc., 1995, 117, 7245
Zeng W., Zeng G. Y., Qin S. Y., Chin. J. Org. Chem., 2003, 23(11), 1213
Jiao J., Fang H., Wang X. J., Guan P., Yuan Y. M., Xu W. F., Eur. J. Med. Chem., 2009, 44, 4470
Li H. B., Qin C., Liang W., Wang L. P., Hao M. A., Jin B., Liang X. L., Qin S.Y., J. Mol. Catal., 2009, 23(1), 62
Helmick J. S., Martin K. A., Heinrich J. L., J. Am. Chem. Soc., 1991, 113, 3459
Raymond K. N., Coord. Chem. Rev., 1990, 105, 135
Busch D. H., Alcock N. W., Chem. Rev., 1994, 94, 585
Li G. Q., Govind R., Ind. Eng. Chem. Res., 1994, 33, 755
Zhang S. Y., Jiang P. P., Leng Y., Xu Y. C., Mo G. T., Bian G., Chem. J. Chinese Universities, 2013, 34(7), 1703
Mukherjee M., Ray A. R., J. Mol. Catal. A, 2007, 266(1/2), 207
Han Z. J., Zhou H., Chen H. W., Dai H., J. Inorg. Chem., 1992, 8(4), 421
Yang H., Qin S. Y., Lu X. X., Zeng W., Chin. Chem. Lett., 1999, 10, 79
Li H. B., Du Y., Wei X. Y., Qin S. Y., Acta Chim. Sinica, 2002, 60(5), 886
Barlan A. U., Zhang W., Yamamoto H., Tetrahedron, 2007, 63(27), 6075
Gharah N., Chattopadhyay B., Maiti S. K., Mukherjee M., Transition Met. Chem., 2010, 35(5), 531
Sun B., Qin S. Y., Chem. Res. Appl.(China), 2011, 23(1), 92
Li Y. F., Gao B. J., Yu Y. L., J. Mol. Catal.(China), 2013, 27(3), 271
Wang R. X., Zhu H. L., Gao B. J., Reac. Kinet. Mech. Cat., 2011, 103, 431
Barrio L., Toribio P. P., Campos-Martin J. M., Fierro J. L. G., Tetrahedron, 2004, 60, 11527
Lü C. L., Gao B. J., Liu Q., Qi C. S., Colloid Polym. Sci., 2008, 286, 553
Alcántara R., Canoira L., Joao P. G., Santos J. M., Vázquez I., Appl. Catal. A, 2000, 203, 259
Agrawal D. R., Tandon S. G., J. Indian Chem. Soc., 1971, 48, 571
Toribio P. P., Campos-Martin J. M., Fierro J. L. G., J. Mol. Catal. A, 2005, 227, 101
Lu Z. F., Zhan F. T., Qin S. Y., Modern Chemical Industry, 2000, 20(5), 32
Toribio P. P., Campos-Martin J. M., Fierro J. L. G., Appl. Catal. A, 2005, 294, 290
Li H. B., Qin C., Yang W. B., Hu X. P., Qin S. Y., Chin. Chem. Lett., 2007, 18, 103
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Young Scientists Fund of China(No.21307116), the Natural Science Foundation of Shanxi Province, China(No.2014011017-5), the Project for the Outstanding Innovative Teams of Higher Learning Institutions of Shanxi Province, China(No.201504) and the Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province, China(No.20140828).
Rights and permissions
About this article
Cite this article
Wang, R., Wang, H., Wang, X. et al. Immobilization of transition-metal hydroxamates on polystyrene resins: Effective biomimetic heterogeneous catalysts for aerobic oxidation of ethylbenzene. Chem. Res. Chin. Univ. 31, 835–840 (2015). https://doi.org/10.1007/s40242-015-5072-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-015-5072-8