Abstract
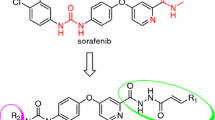
A series of hydrazine and oxadiazole analogs of Sorafenib was designed, synthesized and characterized by proton nuclear magnetic resonance(1H NMR) spectrometry and high resolution mass spectrometry(HRMS). The antiproliferative activities of these compounds against human colorectal carcinoma(HCT-116) and human breast cancer (MDA-MB-231) tumor cell lines were evaluated in vitro by MTT method[MTT=3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]. The bioassay results suggest that most of the synthesized compounds have antitumor potential to HCT-116 cell line compared with MDA-MB-231 cell line. Compounds 8a, 8b, 8d, 8e, 9f and 9j competitive with Sorafenib demonstrated antiproliferative activities on HCT-116 cell line.
Similar content being viewed by others
References
Smalley K. S., Haass N. K., Brafford P. A., Lioni M., Flaherty K. T., Herlyn M., Mol. Cancer Ther., 2006, 5, 1136
Sergina N. V., Rausch M., Wang D., Blair J., Hann B., Shokat K. M., Moasser M. M., Nature, 2007, 445, 437
Balz L. M., Bartkowiak K., Andreas A., Pantel K., Niggemann B., Zanker K. S., Brandt B. H., Dittmar T., J. Pathol., 2012, 227, 234
Vazquez S., Leon L., Fernandez O., Lazaro M., Grande E., Aparicio L., Adv. Ther., 2012, 29, 202
Gild M. L., Bullock M., Robinson B. G., Clifton-Bligh R., Nat. Rev. Endocrinol., 2011, 7, 617
Ranieri G., Gadaleta-Caldarola G., Goffredo V., Patruno R., Mangia A., Rizzo A., Sciorsci R. L., Gadaleta C. D., Curr. Med. Chem., 2012, 19, 938
Yao J., Chen J., He Z., Sun W., Xu W., Bioorg. Med. Chem., 2012, 20, 2923
Sun M., Wu X., Chen J., Cai J., Cao M., Ji M., Eur. J. Med. Chem., 2010, 45, 2299
Kumar D., Patel G., Chavers A. K., Chang K. H., Shah K., Eur. J. Med. Chem., 2011, 46, 3085
Boström J., Hogner A., Llinàs A., Wellner E., Plowright A. T., J. Med. Chem., 2012, 55, 1817
Singh N. K., Singh N., Prasad G. C., Sodhi A., Shrivastava A., Bioorg. Med. Chem., 1997, 5, 245
Mohareb R. M., Fleita D. H., Sakka O. K., Molecules, 2011, 16, 16
Wilhelm S. M., Carter C., Tang L., Wilkie D., McNabola A., Rong H., Chen C., Zhang X., Vincent P., McHugh M., Cao Y., ShuJath J., Gawlak S., Eveleigh D., Rowley B., Liu L., Adnane L., Lynch M., Auclair D., Taylor I., Gedrich R., Voznesensky A., Riedl B., Post L. E., Bollag G., Trail P. A., Cancer Res., 2004, 64, 7099
Dydio P., Zieliński T., Jurczak J., J. Org. Chem., 2009, 74, 1525
Rajapakse H. A., Zhu H., Young M. B., Mott B. T., Tetrahedron Lett., 2006, 47, 4827
Liu P., Zhang J., Yan T., Xiong L., Li. Z., Chem. Res. Chinese Universities, 2012, 28(3), 430
Riedl B., Dumas J., Khire U., Lowinger T. B., Scott W. L., Smith R. A., Wood J. E., Monahan M. K., Natero R., Renick J., Sibley R. N., Omega-Carboxyaryl Substituted Diphenyl Ureas as Raf Kinase Inhibitiors, US7235576 B1, 2007
Deegan T. L., Nitz T. J., Cebzanov D., Pufko D. E., Porco J. A., Bioorg. Med. Chem. Lett., 1999, 9, 209
Hu G., Wang G., Duan N., Wen X., Cao T., Xie S., Huang W., Chem. Res. Chinese Universities, 2012, 28(6), 980
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the National Natural Science Foundation of China(No.21072115) and the Student Training Programs for Innovation of Shandong University, China(No.201210422076).
Rights and permissions
About this article
Cite this article
Wang, Yy., Liu, Jz., Yu, Xy. et al. Design and synthesis of hydrazine and oxadiazole-containing derivatives of Sorafenib as antitumor agents. Chem. Res. Chin. Univ. 29, 454–459 (2013). https://doi.org/10.1007/s40242-013-2490-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-013-2490-3