Abstract
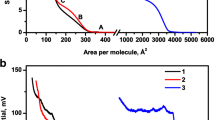
Compression isotherm for stearic acid was obtained by means of molecular dynamic simulation and compared to experimentally measured values for the Langmuir monolayers. Compared to the previous simulation, the present simulation has provided a method to reproduce the compression of the monolayer. The result is consistent with other experimental results. By analyzing the alkyl tails, the configuration of stearic acid molecules during the compression process was studied and a uniform monolayer was obtained after compression. Stearic acid molecules were observed to form fine organized monolayer from completely random structure. Hexatic order of the arrangement has been identified for the distribution of stearic acid molecules in the monolayer. At the end of the compression, the stearic acid molecules were tightly packed in the gap of two other molecules. At last, the hydrogen bonds in the system were analyzed. The main hydrogen bonds were from stearic acid-water interaction and their intensities constantly decreased with the decreased of surface area per molecule. The weak hydrogen bond interaction between stearic acid molecules may be the reason of easy collapse.
Similar content being viewed by others
Reference
Sanassy P., Evans S. D., Langmuir, 1993, 9, 1024
Cayci D., Stanciu S. G., Capan I., Erdogan M., Guner B., Hristu R., Stanciu G. A., Sensor Actuat B, Chem., 2011, 158, 62
Graf K., Riegler H., Colloids Surf. A, 1998, 131, 215
Petty M. C., Langmuir-Blodgett Films: An Introduction, Cambridge University Press, Cambridge, 1996
George L. Gaines, Insoluble Monolayers at Liquid-gas Interface, Interscience, New York, 1966
Cao Z., Chen Q., Wang C., Ren Y., Liu H., Hu Y., Colloids Surf. A, 2011, 377, 130
Song E., Shi C., Anson F. C., Langmuir, 1998, 14, 4315
Dunbar A. D. F., Richardson T. H., McNaughton A. J., Hutchinson J., Hunter C. A., J. Phys. Chem. B, 2006, 110, 16646
Schmidtke J. P., Friend R. H., Kastler M., Mullen K., J. Chem. Phys., 2006, 124, 174704
Ferreira M., Constantino C. J. L., Olivati C. A., Balogh D. T., Aroca R. F., Faria R. M., Oliveira O. N. Jr., Polymer, 2005, 46, 5140
de Luca G., Pollicino G., Romeo A., Scolaro L. M., Chem. Mater., 2006, 18, 2005
Rana A. F., Frank J. M., J. Chem. Phys., 1973, 59, 5529
Ignes-Mullol J., Claret J., Reigada R., Sagues F., Phys. Rep., 2007, 448, 163
Tanaka H., Kunai Y., Sato N., Matsuyama T., J. Colloid Interface Sci., 2011, 363, 440
Su A., Tan S., Thapa P., Flanders B. N., Ford W. T., J. Phys. Chem. C, 2007, 111, 4695
Ghaicha L., Chattopadhyay A. K., Tajmir-Riahi H. A., Langmuir, 1991, 7, 2007
Yazdanian M., Yu H., Zografi G., Langmuir, 1990, 6, 1093
Hwang M. J., Kim K., Langmuir, 1999, 15, 3563
Munden J. W., Blois D. W., Swarbrick J., J. Pharm. Sci., 1969, 58, 1308
Houmadi S., Ciuchi F., de Santo M. P., de Stefano L., Rea I., Giardina P., Armenante A., Lacaze E., Giocondo M., Langmuir, 2008, 24, 12953
Nayak A., Suresh K. A., Kumar Pal S., Kumar S., J. Phys. Chem. B, 2007, 111, 11157
Liu M. T., Pu M. F., Ma H. W., Chem. J. Chinese Universities, 2012, 33(6), 1319
Moghaddam B., Ali M. H., Wilkhu J., Kirby D. J., Mohammed A. R., Zheng Q., Perrie Y., Int. J. Pharm., 2011, 417, 235
Shi W. X., Guo H. X., J. Phys. Chem. B, 2010, 114, 6365
Javanainen M., Monticelli L., de la Serna J. B., Vattulainen I., Langmuir, 2010, 26, 15436
Henry D. J., Dewan V. I., Prime E. L., Qiao G. G., Solomon D. H., Yarovsky I., J. Phys. Chem. B, 2010, 114, 3869
Giner-Casares J. J., Camacho L., Martin-Romero M. T., Lopez Cascales J. J., Langmuir, 2008, 24, 1823
Knobler C. M., Advances in Chemical Physics, John Wiley & Sons, Inc., New York, 2007, 397
Langmuir I., J. Am. Chem. Soc., 1917, 39, 1848
Griffith E. C., Adams E. M., Allen H. C., Vaida V., J. Phys. Chem. B, 2012, 116, 7849
Shaitan K. V., Pustoshilov P. P., Biophysics, 1991, 44, 429
Okamura E., Fukushima N., Hayashi S., Langmuir, 1999, 15, 3589
Lee Y. L., Yang Y. C., Shen Y. J., J. Phys. Chem. B, 2005, 109, 4662
Moller M. A., Tildesley D. J., Kim K. S., Quirke N., J. Chem. Phys., 1991, 94, 8390
Berendsen H. J. C., Postma J. P. M., van Gunsteren W. F., Hermans J., Intermolecular Forces, 1981, 331
Feng C., Paul E. S., J. Chem. Phys., 2007, 126, 221101
Abascal J. L. F., Vega C., J. Chem. Phys., 2005, 123, 234505
Jose A., Gustavo A. C., J. Chem. Phys., 2010, 132, 014701
Jorgensen W. L., Maxwell D. S., Tirado Rives J., J. Am. Chem. Soc., 1996, 118, 11225
Jorgensen W. L., Chandrasekhar J., Madura J. D., Impey R. W., Klein M. L., J. Chem. Phys., 1983, 79, 926
Berendsen H. J. C., Vanderspoel D., Vandrunen R., Comput. Phys. Commun., 1995, 91, 43
Lindahl E., Hess B., van der Spoel D., J. Mol. Model., 2001, 7, 306
van der Spoel D., Lindahl E., Hess B., Groenhof G., Mark A. E., Berendsen H. J. C., J. Comput. Chem., 2005, 26, 1701
Hess B., Kutzner C., van der Spoel D., Lindahl E., J. Chem. Theory Comput., 2008, 4, 435
Tom D., Darrin Y., Lee P., J. Chem. Phys., 1993, 98, 10089
Bussi G., Donadio D., Parrinello M., J. Chem. Phys., 2007, 126, 014101
Harris J. G., J. Phys. Chem., 1992, 96, 5077
Kaminski G. A., Friesner R. A., Tirado Rives J., Jorgensen W. L., J. Phys. Chem. B, 2001, 105, 6474
Marcus G. M., Fluid Phase Equilib., 2006, 248, 50
Humphrey W., Dalke A., Schulten K., J. Mol. Graphics Modell, 1996, 14, 33
Ghosh S., Il Nuovo Cimento D, 1984, 4, 229
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China(No.20973076) and the Fund of China Scholarship Council(No. 2010617085).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kong, Cp., Zhang, Hx., Zhao, Zx. et al. Molecular dynamic studies on Langmuir monolayers of stearic acid. Chem. Res. Chin. Univ. 29, 545–550 (2013). https://doi.org/10.1007/s40242-013-2301-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-013-2301-x