Abstract
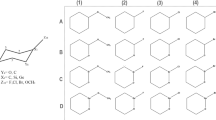
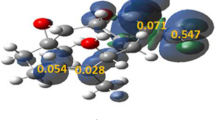
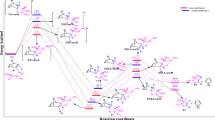
A mechanism about the origin of the selectivities for the cleavage of dioxolane five-membered rings on pyranoside rings was suggested. Quantum chemical studies were performed to testify the rationality of the mechanism. It is thus suggested that the selectivities should be dependent on the differences of the free energy at the transition states when the five-membered ring cleaves. Natural bond orbital(NBO) analysis was further made to assess the influence of stereoelectronic effects on the selectivities.
Similar content being viewed by others
References
Binkley R. W., Goewey G. S., Johnston J. C., J. Org. Chem., 1984, 49(6), 992
Deslongchamps P., Tetrahedron, 1975, 31(20), 2463
King J. F., Allbutt A. D., Can. J. Chem., 1970, 48(11), 1754
Deslongchamps P., Chênevert R., Taillefer R. J., Moreau C., Saunders J. K., Can. J. Chem., 1975, 53(11), 1601
Dong H., Pei Z. C., Ramström O., Chem. Commun., 2008, 1359
Dong H., Pei Z. C., Ramström O., J. Org. Chem., 2006, 71(8), 3306
Dong H., Pei Z. C., Angelin M., Byström S., Ramström O., J. Org. Chem., 2007, 72(10), 3694
Binkley R. W., J. Org. Chem., 1991, 56(12), 3892
Deng S. L., Gangadharmath U., Chang C. W. T., J. Org. Chem., 2006, 71(14), 5179
Deng S. L., Chang C. W. T., Synlett., 2006, 756
Chittenden G. J. F., Buchanan J. G., Carbohydr. Res., 1969, 11(3), 379
Perrin C. L., Acc. Chem. Res., 2002, 35(1), 28
Knapp S., Kukkola P. J., Sharma S., Dhar T. G. M., Naughton A. B. J., J. Org. Chem., 1990, 55(22), 5700
Knapp S., Naughton A. B. J., Jaramillo C., Pipik B., J. Org. Chem., 1992, 57(26), 7328
Wang H. S., She J., Zhang L. H., Ye X. S., J. Org. Chem., 2004, 69(17), 5774
Hu G. X., Vasella A., Helv. Chim. Acta, 2002, 85(12), 4369
Carey F. A., Sundberg R. J., Advanced Organic Chemistry, Kluwer Academic/Plenum Publishers, New York, 2000, 157
Ding X. Q., Ding J. J., Li D. Y., Sun Y., Chen J. S., Chem. J. Chinese Universities, 2012, 33(5), 1063
Sang Y. M., Yan L. K., Wen S. Z., Cong S., Su Z. M., Chem. J. Chinese Universities, 2012, 33(6), 1285
Wan S. Q., Zhang Y., Zhang H., Sun C. C., Chem. J. Chinese Universities, 2012, 33(7), 1505
Wang Y., Zhang P., Yang K., Hou Y. M., Chem. J. Chinese Universities, 2012, 33(8), 1799
Liu N. N., Ding Y. H., Chem. J. Chinese Universities, 2012, 33(9), 2043
Fan L. T., Li Y., Wu D., Li Z. R., Sun C. C., Chem. J. Chinese Universities, 2012, 33(10), 2320
Chai J. D., Head-Gordon M., Phys. Chem. Chem. Phys., 2008, 10(44), 6615
Cossi M., Rega N., Scalmani G., Barone V., J. Comp. Chem., 2003, 24(6), 669
Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A. Jr., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam N. J., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas Ö., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision C.01, Gaussian Inc., Wallingford CT, 2009
Glendening E. D., Reed A. E., Carpenter J. E., Weinhold F., NBO 3.1, Theoretical Chemistry Institute, University of Wisconsin, Madison WI, 2001
Alabugin I. V., Manoharan M., Zeidan T. A., J. Am. Chem. Soc., 2003, 125(46), 14014
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the National Natural Science Foundation of China(No.20973077), the Program for New Century Excellent Talents in University of China(No.NCET-07-0358) and the Brain Gain Fund of Huazhong University of Science and Technology, China.
Rights and permissions
About this article
Cite this article
Pan, Xl., Zhou, Yx., Liu, W. et al. Stereoelectronic control of cleavage of dioxolane five-membered ring on carbohydrates. Chem. Res. Chin. Univ. 29, 551–555 (2013). https://doi.org/10.1007/s40242-013-2293-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-013-2293-6