Abstract
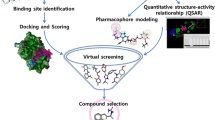
The inhibition of abnormal amyloid β (Aβ) aggregation has been regarded as a good target to control Alzheimer’s disease. The present study adopted 2D-QSAR, HQSAR and 3D QSAR (CoMFA & CoMSIA) modeling approaches to identify the structural and physicochemical requirements for the potential Aβ aggregation inhibition. A structure-based molecular docking technique is utilized to approve the features that are obtained from the ligand-based techniques on 30 curcumin derivatives. The combined outputs were then used to screen the modified 10 compounds. The 2D QSAR model on curcumin derivatives gave statistical values R2 = 0.9086 and SEE = 0.1837. The model was further confirmed by Y-randomization test and Applicability domain analysis by the standardization approach. The HQSAR study (Q2 = 0.615, R 2ncv = 0.931, R 2pred = 0.956) illustrated the important molecular fingerprints for inhibition. Contour maps of 3D QSAR models, CoMFA (Q2 = 0.687, R 2ncv = 0.787, R 2pred = 0.731) and CoMSIA (Q2 = 0.743, R 2ncv = 0.972, R 2pred = 0.713), depict that the models are robust and provide explanation of the important features, like steric, electrostatic and hydrogen bond acceptor, which play important role for interaction with the receptor site cavity. The molecular docking study of the curcumin derivatives elucidates the important interactions between the amino acid residues at the catalytic site of the receptor and the ligands, indicating the structural requirements of the inhibitors. The ligand–receptor interactions of top hits were analyzed to explore the pharmacophore features of Aβ aggregation inhibition. The Aβ aggregation inhibitory activities of novel chemical entities were then obtained through inverse QSAR. The newly designed molecules were further screened through machine learning, prediction of toxicity and nature of metabolism to get the proposed six lead compounds.








Similar content being viewed by others
References
Ajay, Bemis GW, Murcko MA (1999) Designing libraries with CNS activity. J Med Chem 42:4942–4951. https://doi.org/10.1021/jm990017w
Alzheimer’s Association (2017) 2017 Alzheimer’s disease facts and figures. Alzheimer’s Dement 13:325–373. https://doi.org/10.1016/j.jalz.2017.02.001
Aswathy L, Jisha RS, Masand VH et al (2017) Computational strategies to explore antimalarial thiazine alkaloid lead compounds based on an Australian marine sponge Plakortis lita. J Biomol Struct Dyn 35:2407–2429. https://doi.org/10.1080/07391102.2016.1220870
Ballante F, Ragno R (2012) 3-D QSAutogrid/R: an alternative procedure to build 3-D QSAR models. methodologies and applications. J Chem Inf Model 52:1674–1685. https://doi.org/10.1021/ci300123x
Begum AN, Jones MR, Lim GP et al (2008) Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J Pharmacol Exp Ther 326:196–208. https://doi.org/10.1124/jpet.108.137455
Caesar I, Jonson M, Nilsson KPR et al (2012) Curcumin promotes A-beta fibrillation and reduces neurotoxicity in transgenic drosophila. PLoS ONE 7:e31424. https://doi.org/10.1371/journal.pone.0031424
Chatake T, Tanaka I, Umino H et al (2005) The hydration structure of a Z-DNA hexameric duplex determined by a neutron diffraction technique. Acta Crystallogr D Biol Crystallogr 61:1088–1098. https://doi.org/10.1107/S0907444905015581
Citron M (2010) Alzheimer’s disease: strategies for disease modification. Nat Rev Drug Discov 9:387–398. https://doi.org/10.1038/nrd2896
Cramer RD, Patterson DE, Bunce JD (1988) Comparative molecular field analysis (CoMFA). 1. Effect of shape on binding of steroids to carrier proteins. J Am Chem Soc 110:5959–5967. https://doi.org/10.1021/ja00226a005
Cruciani G, Watson KA (1994) Comparative molecular field analysis using GRID force-field and GOLPE variable selection methods in a study of inhibitors of glycogen phosphorylase b. J Med Chem 37:2589–2601. https://doi.org/10.1021/jm00042a012
Dong M, Lu X, Ma Y et al (2015) An efficient approach for automated mass segmentation and classification in mammograms. J Digit Imaging 28:613–625. https://doi.org/10.1007/s10278-015-9778-4
Dubey SK, Sharma AK, Narain U et al (2008) Design, synthesis and characterization of some bioactive conjugates of curcumin with glycine, glutamic acid, valine and demethylenated piperic acid and study of their antimicrobial and antiproliferative properties. Eur J Med Chem 43:1837–1846. https://doi.org/10.1016/j.ejmech.2007.11.027
Elfiky AA, Elshemey WM (2016) IDX-184 is a superior HCV direct-acting antiviral drug: a QSAR study. Med Chem Res 25:1005–1008. https://doi.org/10.1007/s00044-016-1533-y
Frank E, Hall M, Holmes G et al (2005) Weka. In: Maimon O, Rokach L (eds) Data mining and knowledge discovery handbook. Springer, Boston, MA, pp 1305–1314
Garcia-Alloza M, Borrelli LA, Rozkalne A et al (2007) Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model: curcumin reverses amyloid pathology in vivo. J Neurochem 102:1095–1104. https://doi.org/10.1111/j.1471-4159.2007.04613.x
Gasteiger E, Hoogland C, Gattiker A et al (2005) Protein identification and analysis tools on the ExPASy server. In: Walker JM (ed) The proteomics protocols handbook. Humana Press, Totowa, pp 571–607
Geourjon C, Deléage G (1995) SOPMA: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput Appl Biosci 11:681–684
Gibbs MA, Hosea NA (2003) Factors affecting the clinical development of cytochrome P450 3A substrates. Clin Pharmacokinet 42:969–984. https://doi.org/10.2165/00003088-200342110-00003
Gill SC, von Hippel PH (1989) Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem 182:319–326. https://doi.org/10.1016/0003-2697(89)90602-7
Golbraikh A, Tropsha A (2002) Beware of q2! J Mol Graph Model 20:269–276
Golde TE, Eckman CB, Younkin SG (2000) Biochemical detection of Abeta isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer’s disease. Biochim Biophys Acta 1502:172–187
Guruprasad K, Reddy BV, Pandit MW (1990) Correlation between stability of a protein and its dipeptide composition: a novel approach for predicting in vivo stability of a protein from its primary sequence. Protein Eng 4:155–161
Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol 8:101–112. https://doi.org/10.1038/nrm2101
Halgren TA (1996) Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J Comput Chem 17:490–519
Hamaguchi T, Ono K, Murase A, Yamada M (2009) Phenolic compounds prevent Alzheimer’s pathology through different effects on the amyloid-β aggregation pathway. Am J Pathol 175:2557–2565. https://doi.org/10.2353/ajpath.2009.090417
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356. https://doi.org/10.1126/science.1072994
Hsu J-L, Hung P-C, Lin H-Y, Hsieh C-H (2015) Applying under-sampling techniques and cost-sensitive learning methods on risk assessment of breast cancer. J Med Syst. https://doi.org/10.1007/s10916-015-0210-x
Ikai A (1980) Thermostability and aliphatic index of globular proteins. J Biochem 88:1895–1898
Jack CR, Knopman DS, Jagust WJ et al (2010) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9:119–128. https://doi.org/10.1016/S1474-4422(09)70299-6
Janitza S, Strobl C, Boulesteix A-L (2013) An AUC-based permutation variable importance measure for random forests. BMC Bioinform 14:119. https://doi.org/10.1186/1471-2105-14-119
Jin W, Wang J, Zhu T et al (2014) Anti-inflammatory effects of curcumin in experimental spinal cord injury in rats. Inflamm Res 63:381–387. https://doi.org/10.1007/s00011-014-0710-z
Jisha RS, Aswathy L, Masand VH et al (2017) Exploration of 3,6-dihydroimidazo(4,5-d)pyrrolo(2,3-b)pyridin-2(1H)-one derivatives as JAK inhibitors using various in silico techniques. In Silico Pharmacol. https://doi.org/10.1007/s40203-017-0029-x
Kapetanovic IM (2008) Computer-aided drug discovery and development (CADDD): in silico-chemico-biological approach. Chem Biol Interact 171:165–176. https://doi.org/10.1016/j.cbi.2006.12.006
Klebe G, Abraham U, Mietzner T (1994) Molecular similarity indices in a comparative analysis (CoMSIA) of drug molecules to correlate and predict their biological activity. J Med Chem 37:4130–4146. https://doi.org/10.1021/jm00050a010
Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105–132
Lengauer T, Rarey M (1996) Computational methods for biomolecular docking. Curr Opin Struct Biol 6:402–406
Lim GP, Chu T, Yang F et al (2001) The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci 21:8370–8377
Lührs T, Ritter C, Adrian M et al (2005) 3D structure of Alzheimer’s amyloid-beta(1-42) fibrils. Proc Natl Acad Sci USA 102:17342–17347. https://doi.org/10.1073/pnas.0506723102
Ma X, Chen C, Yang J (2005) Predictive model of blood–brain barrier penetration of organic compounds. Acta Pharmacol Sin 26:500–512. https://doi.org/10.1111/j.1745-7254.2005.00068.x
Ma Q-L, Zuo X, Yang F et al (2013) Curcumin suppresses soluble tau dimers and corrects molecular chaperone, synaptic, and behavioral deficits in aged human tau transgenic mice. J Biol Chem 288:4056–4065. https://doi.org/10.1074/jbc.M112.393751
Mannu J, Jenardhanan P, Mathur PP (2011) A computational study of CYP3A4 mediated drug interaction profiles for anti-HIV drugs. J Mol Model 17:1847–1854. https://doi.org/10.1007/s00894-010-0890-6
Negi PS, Jayaprakasha GK, Jagan Mohan Rao L et al (1999) Antibacterial activity of turmeric oil: a byproduct from curcumin manufacture. J Agric Food Chem 47:4297–4300. https://doi.org/10.1021/jf990308d
Nguyen TKC, Dzung TTK, Cuong PV (2014) Assessment of antifungal activity of turmeric essential oil-loaded chitosan nanoparticles. J Chem Bio Phy Sci Sec B 4:2347–2356
Nishikawa H, Tsutsumi J, Kitani S (2013) Anti-inflammatory and anti-oxidative effect of curcumin in connective tissue type mast cell. J Funct Foods 5:763–772. https://doi.org/10.1016/j.jff.2013.01.022
Ojha PK, Roy K (2011) Comparative QSARs for antimalarial endochins: importance of descriptor-thinning and noise reduction prior to feature selection. Chemom Intell Lab Syst 109:146–161. https://doi.org/10.1016/j.chemolab.2011.08.007
Ono K, Hasegawa K, Naiki H, Yamada M (2004) Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J Neurosci Res 75:742–750. https://doi.org/10.1002/jnr.20025
Roy K, Kar S, Ambure P (2015a) On a simple approach for determining applicability domain of QSAR models. Chemom Intell Lab Syst 145:22–29. https://doi.org/10.1016/j.chemolab.2015.04.013
Roy K, Kar S, Ambure P (2015b) On a simple approach for determining applicability domain of QSAR models. Chemom Intell Lab Syst 145:22–29. https://doi.org/10.1016/j.chemolab.2015.04.013
Sajeev R, Athira RS, Nufail M et al (2013) Computational predictive models for organic semiconductors. J Comput Electron 12:790–795. https://doi.org/10.1007/s10825-013-0486-3
Saleh NA (2015) The QSAR and docking calculations of fullerene derivatives as HIV-1 protease inhibitors. Spectrochim Acta Part A Mol Biomol Spectrosc 136:1523–1529. https://doi.org/10.1016/j.saa.2014.10.045
Seal A, Passi A, Jaleel UA et al (2012) In-silico predictive mutagenicity model generation using supervised learning approaches. J Cheminform 4:10. https://doi.org/10.1186/1758-2946-4-10
Selkoe DJ (1994) Cell biology of the amyloid beta-protein precursor and the mechanism of Alzheimer’s disease. Annu Rev Cell Biol 10:373–403. https://doi.org/10.1146/annurev.cb.10.110194.002105
Selkoe DJ (1997) Alzheimer’s disease: genotypes, phenotypes, and treatments. Science 275:630–631
Sharma RA, McLelland HR, Hill KA et al (2001) Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res 7:1894–1900
Shibi IG, Aswathy L, Jisha RS et al (2015) Molecular docking and QSAR analyses for understanding the antimalarial activity of some 7-substituted-4-aminoquinoline derivatives. Eur J Pharm Sci 77:9–23. https://doi.org/10.1016/j.ejps.2015.05.025
Shibi IG, Aswathy L, Jisha RS et al (2016) Virtual screening techniques to probe the antimalarial activity of some traditionally used phytochemicals. Comb Chem High Throughput Screen 19:572–591
Tetko IV, Tanchuk VY, Villa AE (2001) Prediction of n-octanol/water partition coefficients from PHYSPROP database using artificial neural networks and E-state indices. J Chem Inf Comput Sci 41:1407–1421
Tropsha A, Gramatica P, Gombar V (2003) The importance of being earnest: validation is the absolute essential for successful application and interpretation of QSPR models. QSAR Comb Sci 22:69–77. https://doi.org/10.1002/qsar.200390007
Wahi D, Jamal S, Goyal S et al (2015) Cheminformatics models based on machine learning approaches for design of USP1/UAF1 abrogators as anticancer agents. Syst Synth Biol 9:33–43. https://doi.org/10.1007/s11693-015-9162-1
Xiao Y, Ma B, McElheny D et al (2015) Aβ(1–42) fibril structure illuminates self-recognition and replication of amyloid in Alzheimer’s disease. Nat Struct Mol Biol 22:499–505. https://doi.org/10.1038/nsmb.2991
Yamashita S, Furubayashi T, Kataoka M et al (2000) Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur J Pharm Sci 10:195–204
Yanagisawa D, Taguchi H, Morikawa S et al (2015) Novel curcumin derivatives as potent inhibitors of amyloid β aggregation. Biochem Biophys Rep 4:357–368. https://doi.org/10.1016/j.bbrep.2015.10.009
Yang F, Lim GP, Begum AN et al (2005a) Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem 280:5892–5901. https://doi.org/10.1074/jbc.M404751200
Yang H, Xie W, Xue X et al (2005b) Design of wide-spectrum inhibitors targeting coronavirus main proteases. PLoS Biol 3:e324. https://doi.org/10.1371/journal.pbio.0030324
Yap CW (2011) PaDEL-descriptor: an open source software to calculate molecular descriptors and fingerprints. J Comput Chem 32:1466–1474. https://doi.org/10.1002/jcc.21707
Zhao YH, Le J, Abraham MH et al (2001) Evaluation of human intestinal absorption data and subsequent derivation of a quantitative structure-activity relationship (QSAR) with the Abraham descriptors. J Pharm Sci 90:749–784
Acknowledgements
Aswathy L. is thankful to CSIR, New Delhi for the financial assistance in the form of Senior Research Fellowship. Jisha, R.S. is thankful to the University of Kerala, Thiruvananthapuram for providing financial assistance in the form of University Junior Research Fellowship for this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aswathy, L., Jisha, R.S., Masand, V.H. et al. Design of novel amyloid β aggregation inhibitors using QSAR, pharmacophore modeling, molecular docking and ADME prediction. In Silico Pharmacol. 6, 12 (2018). https://doi.org/10.1007/s40203-018-0049-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40203-018-0049-1