Abstract
Background
Perfluoroalkyl and polyfluoroalkyl substances (PFASs) are persistent organic pollutants in the environment. While some studies suggest that PFASs may contribute to cancer development, the link between PFAS exposure and cancer risk remains debated.
Methods
This dose-response meta-analysis explores the relationship between PFASs and cancer. It employs odds ratio (OR) and standardized mean difference (SMD), along with their 95% confidence interval (CI), to assess the effects of PFASs on cancer risk. Relevant studies were sourced from Web of Science, PubMed, Embase, Medline, and CNKI databases. The dose-response relationship was assessed by the fixed-effects model and least-squares regression.
Results
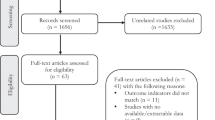
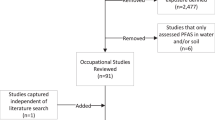
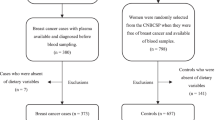
Forty studies, involving a total of 748,188 participants, were included in this meta-analysis. Out of these, 13 studies were specifically analyzed for the dose-response relationship. Findings revealed that exposure to PFASs, especially PFDA, significantly raises the risk of genitourinary cancers, and PFDA exposure shows a dose-dependent increase in overall and breast cancer risk. Additionally, PFOS exposure is associated with an increased cancer risk, and elevated PFOA levels were significantly observed in breast cancer patients.
Conclusions
The findings suggest that PFAS exposure is a potential cancer risk factor, with the carcinogenic potential of PFDA being dose-dependent.




Similar content being viewed by others
Data availability
All data are incorporated into the article and its online supplementary material.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. https://doi.org/10.3322/caac.21708.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. https://doi.org/10.3322/caac.21338.
Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366(9499):1784–93. https://doi.org/10.1016/s0140-6736(05)67725-2.
Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: global burden of disease study. Lancet. 1997;349(9063):1436–42. https://doi.org/10.1016/s0140-6736(96)07495-8.
Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol. 2017;13(3):161–73. https://doi.org/10.1038/nrendo.2016.186.
De Silva AO, Armitage JM, Bruton TA, Dassuncao C, Heiger-Bernays W, Hu XC, Kärrman A, Kelly B, Ng C, Robuck A, et al. PFAS exposure pathways for humans and wildlife: a synthesis of current knowledge and key gaps in understanding. Environ Toxicol Chem. 2021;40(3):631–57. https://doi.org/10.1002/etc.4935.
Domingo JL, Nadal M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: a review of the recent scientific literature. Environ Res. 2019;177:108648. https://doi.org/10.1016/j.envres.2019.108648.
Sen P, Qadri S, Luukkonen PK, Ragnarsdottir O, McGlinchey A, Jäntti S, Juuti A, Arola J, Schlezinger JJ, Webster TF, et al. Exposure to environmental contaminants is associated with altered hepatic lipid metabolism in non-alcoholic fatty liver disease. J Hepatol. 2022;76(2):283–93. https://doi.org/10.1016/j.jhep.2021.09.039.
Wang Z, Cousins IT, Scheringer M, Buck RC, Hungerbühler K. Global emission inventories for C4-C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, part I: production and emissions from quantifiable sources. Environ Int. 2014;70:62–75. https://doi.org/10.1016/j.envint.2014.04.013.
Clarke DB, Bailey VA, Routledge A, Lloyd AS, Hird S, Mortimer DN, Gem M. Dietary intake estimate for perfluorooctanesulphonic acid (PFOS) and other perfluorocompounds (PFCs) in UK retail foods following determination using standard addition LC-MS/MS. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2010;27(4):530–45. https://doi.org/10.1080/19440040903476590.
Domingo JL. Health risks of dietary exposure to perfluorinated compounds. Environ Int. 2012;40:187–95. https://doi.org/10.1016/j.envint.2011.08.001.
Schwanz TG, Llorca M, Farré M, Barceló D. Perfluoroalkyl substances assessment in drinking waters from Brazil. France and Spain Sci Total Environ. 2016;539:143–52. https://doi.org/10.1016/j.scitotenv.2015.08.034.
Haug LS, Thomsen C, Brantsaeter AL, Kvalem HE, Haugen M, Becher G, Alexander J, Meltzer HM, Knutsen HK. Diet and particularly seafood are major sources of perfluorinated compounds in humans. Environ Int. 2010;36(7):772–8. https://doi.org/10.1016/j.envint.2010.05.016.
Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99(2):366–94. https://doi.org/10.1093/toxsci/kfm128.
Wang F, Liu W, Jin Y, Dai J, Yu W, Liu X, Liu L. Transcriptional effects of prenatal and neonatal exposure to PFOS in developing rat brain. Environ Sci Technol. 2010;44(5):1847–53. https://doi.org/10.1021/es902799f.
Wang F, Liu W, Ma J, Yu M, Jin Y, Dai J. Prenatal and neonatal exposure to perfluorooctane sulfonic acid results in changes in miRNA expression profiles and synapse associated proteins in developing rat brains. Environ Sci Technol. 2012;46(12):6822–9. https://doi.org/10.1021/es3008547.
Barry V, Winquist A, Steenland K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect. 2013;121(11-12):1313–8. https://doi.org/10.1289/ehp.1306615.
Wielsøe M, Kern P, Bonefeld-Jørgensen EC. Serum levels of environmental pollutants is a risk factor for breast cancer in Inuit: a case control study. Environ Health. 2017;16(1):56. https://doi.org/10.1186/s12940-017-0269-6.
Omoike OE, Pack RP, Mamudu HM, Liu Y, Wang L. A cross-sectional study of the association between perfluorinated chemical exposure and cancers related to deregulation of estrogen receptors. Environ Res. 2021;196:110329. https://doi.org/10.1016/j.envres.2020.110329.
Shearer JJ, Callahan CL, Calafat AM, Huang WY, Jones RR, Sabbisetti VS, Freedman ND, Sampson JN, Silverman DT, Purdue MP, et al. Serum concentrations of per- and Polyfluoroalkyl substances and risk of renal cell carcinoma. J Natl Cancer Inst. 2021;113(5):580–7. https://doi.org/10.1093/jnci/djaa143.
Cao L, Guo Y, Chen Y, Hong J, Wu J, Hangbiao J. Per−/polyfluoroalkyl substance concentrations in human serum and their associations with liver cancer. Chemosphere. 2022;296:134083. https://doi.org/10.1016/j.chemosphere.2022.134083.
Goodrich JA, Walker D, Lin X, Wang H, Lim T, McConnell R, Conti DV, Chatzi L, Setiawan VW. Exposure to perfluoroalkyl substances and risk of hepatocellular carcinoma in a multiethnic cohort. JHEP Rep. 2022;4(10):100550. https://doi.org/10.1016/j.jhepr.2022.100550.
Liu M, Zhang G, Meng L, Han X, Li Y, Shi Y, Li A, Turyk ME, Zhang Q, Jiang G. Associations between novel and legacy per- and Polyfluoroalkyl substances in human serum and thyroid Cancer: a case and healthy population in Shandong Province. East China Environ Sci Technol. 2022;56(10):6144–51. https://doi.org/10.1021/acs.est.1c02850.
Velarde MC, Chan AFO, Sajo M, Zakharevich I, Melamed J, Uy GLB, Teves JMY, Corachea AJM, Valparaiso AP, Macalindong SS, et al. Elevated levels of perfluoroalkyl substances in breast cancer patients within the greater Manila area. Chemosphere. 2022;286(Pt 1):131545. https://doi.org/10.1016/j.chemosphere.2021.131545.
Itoh H, Harada KH, Kasuga Y, Yokoyama S, Onuma H, Nishimura H, Kusama R, Yokoyama K, Zhu J, Harada Sassa M, et al. Serum perfluoroalkyl substances and breast cancer risk in Japanese women: a case-control study. Sci Total Environ. 2021;800:149316. https://doi.org/10.1016/j.scitotenv.2021.149316.
Innes KE, Wimsatt JH, Frisbee S, Ducatman AM. Inverse association of colorectal cancer prevalence to serum levels of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in a large Appalachian population. BMC Cancer. 2014;14:45. https://doi.org/10.1186/1471-2407-14-45.
Steenland K, Zhao L, Winquist A. A cohort incidence study of workers exposed to perfluorooctanoic acid (PFOA). Occup Environ Med. 2015;72(5):373–80. https://doi.org/10.1136/oemed-2014-102364.
Hurley S, Goldberg D, Wang M, Park JS, Petreas M, Bernstein L, Anton-Culver H, Nelson DO, Reynolds P. Breast cancer risk and serum levels of per- and poly-fluoroalkyl substances: a case-control study nested in the California teachers study. Environ Health. 2018;17(1):83. https://doi.org/10.1186/s12940-018-0426-6.
Bonefeld-Jorgensen EC, Long M, Bossi R, Ayotte P, Asmund G, Krüger T, Ghisari M, Mulvad G, Kern P, Nzulumiki P, et al. Perfluorinated compounds are related to breast cancer risk in Greenlandic Inuit: a case control study. Environ Health. 2011;10:88. https://doi.org/10.1186/1476-069x-10-88.
Feng Y, Bai Y, Lu Y, Chen M, Fu M, Guan X, Cao Q, Yuan F, Jie J, Li M, et al. Plasma perfluoroalkyl substance exposure and incidence risk of breast cancer: a case-cohort study in the Dongfeng-Tongji cohort. Environ Pollut. 2022;306:119345. https://doi.org/10.1016/j.envpol.2022.119345.
Mancini FR, Cano-Sancho G, Gambaretti J, Marchand P, Boutron-Ruault MC, Severi G, Arveux P, Antignac JP, Kvaskoff M. Perfluorinated alkylated substances serum concentration and breast cancer risk: evidence from a nested case-control study in the French E3N cohort. Int J Cancer. 2020;146(4):917–28. https://doi.org/10.1002/ijc.32357.
Wen X, Wang M, Xu X, Li T. Exposure to per- and Polyfluoroalkyl substances and mortality in U.S. adults: a population-based cohort study. Environ Health Perspect. 2022;130(6):67007. https://doi.org/10.1289/ehp10393.
Bonefeld-Jørgensen EC, Long M, Fredslund SO, Bossi R, Olsen J. Breast cancer risk after exposure to perfluorinated compounds in Danish women: a case-control study nested in the Danish National Birth Cohort. Cancer Causes Control. 2014;25(11):1439–48. https://doi.org/10.1007/s10552-014-0446-7.
Vieira VM, Hoffman K, Shin HM, Weinberg JM, Webster TF, Fletcher T. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: a geographic analysis. Environ Health Perspect. 2013;121(3):318–23. https://doi.org/10.1289/ehp.1205829.
Yeung LW, Guruge KS, Taniyasu S, Yamashita N, Angus PW, Herath CB. Profiles of perfluoroalkyl substances in the liver and serum of patients with liver cancer and cirrhosis in Australia. Ecotoxicol Environ Saf. 2013;96:139–46. https://doi.org/10.1016/j.ecoenv.2013.06.006.
Li X, Song F, Liu X, Shan A, Huang Y, Yang Z, Li H, Yang Q, Yu Y, Zheng H, et al. Perfluoroalkyl substances (PFASs) as risk factors for breast cancer: a case-control study in Chinese population. Environ Health. 2022;21(1):83. https://doi.org/10.1186/s12940-022-00895-3.
Chang VC, Rhee J, Berndt SI, Moore SC, Freedman ND, Jones RR, Silverman DT, Gierach GL, Hofmann JN, Purdue MP. Serum perfluorooctane sulfonate and perfluorooctanoate and risk of postmenopausal breast cancer according to hormone receptor status: an analysis in the prostate, lung, colorectal and ovarian Cancer screening trial. Int J Cancer. 2023;153(4):775–82. https://doi.org/10.1002/ijc.34487.
Eriksen KT, Sørensen M, McLaughlin JK, Lipworth L, Tjønneland A, Overvad K, Raaschou-Nielsen O. Perfluorooctanoate and perfluorooctanesulfonate plasma levels and risk of cancer in the general Danish population. J Natl Cancer Inst. 2009;101(8):605–9. https://doi.org/10.1093/jnci/djp041.
Purdue MP, Rhee J, Denic-Roberts H, McGlynn KA, Byrne C, Sampson J, Botelho JC, Calafat AM, Rusiecki J. A nested case-control study of serum per- and Polyfluoroalkyl substances and testicular germ cell tumors among U.S. air Force servicemen. Environ Health Perspect. 2023;131(7):77007. https://doi.org/10.1289/ehp12603.
Madrigal JM, Troisi R, Surcel HM, Öhman H, Kivelä J, Kiviranta H, Rantakokko P, Koponen J, Medgyesi DN, Kitahara CM, et al. Prediagnostic serum concentrations of per- and polyfluoroalkyl substances and risk of papillary thyroid cancer in the Finnish maternity cohort. Int J Cancer. 2024;154(6):979–91. https://doi.org/10.1002/ijc.34776.
Tsai MS, Chang SH, Kuo WH, Kuo CH, Li SY, Wang MY, Chang DY, Lu YS, Huang CS, Cheng AL, et al. A case-control study of perfluoroalkyl substances and the risk of breast cancer in Taiwanese women. Environ Int. 2020;142:105850. https://doi.org/10.1016/j.envint.2020.105850.
Hardell E, Kärrman A, van Bavel B, Bao J, Carlberg M, Hardell L. Case-control study on perfluorinated alkyl acids (PFAAs) and the risk of prostate cancer. Environ Int. 2014;63:35–9. https://doi.org/10.1016/j.envint.2013.10.005.
Zhang T, Fu S, Yu K, Albanes D, Moore SC, Purdue MP, Stolzenberg-Solomon RZ. Nested case-control studies investigating serum Perfluorooctanoate and Perfluorooctane sulfonate levels and pancreatic ductal adenocarcinoma in two cohorts. Environ Health Perspect. 2023;131(10):107702. https://doi.org/10.1289/ehp13208.
Li H, Yang M, Yang J, Seery S, Ma C, Liu Y, Zhang X, Li A, Guo H. Per- and polyfluoroalkyl substances and the associated thyroid cancer risk: a case-control study in China. Chemosphere. 2023;337:139411. https://doi.org/10.1016/j.chemosphere.2023.139411.
Alexander BH, Olsen GW. Bladder cancer in perfluorooctanesulfonyl fluoride manufacturing workers. Ann Epidemiol. 2007;17(6):471–8. https://doi.org/10.1016/j.annepidem.2007.01.036.
Winquist A, Hodge JM, Diver WR, Rodriguez JL, Troeschel AN, Daniel J, Teras LR. Case-cohort study of the association between PFAS and selected cancers among participants in the American Cancer Society's Cancer prevention study II LifeLink cohort. Environ Health Perspect. 2023;131(12):127007. https://doi.org/10.1289/ehp13174.
van Gerwen M, Colicino E, Guan H, Dolios G, Nadkarni GN, Vermeulen RCH, Wolff MS, Arora M, Genden EM, Petrick LM. Per- and polyfluoroalkyl substances (PFAS) exposure and thyroid cancer risk. EBioMedicine. 2023;97:104831. https://doi.org/10.1016/j.ebiom.2023.104831.
Rhee J, Chang VC, Cheng I, Calafat AM, Botelho JC, Shearer JJ, Sampson JN, Setiawan VW, Wilkens LR, Silverman DT, et al. Serum concentrations of per- and polyfluoroalkyl substances and risk of renal cell carcinoma in the multiethnic cohort study. Environ Int. 2023;180:108197. https://doi.org/10.1016/j.envint.2023.108197.
Cathey AL, Nguyen VK, Colacino JA, Woodruff TJ, Reynolds P, Aung MT. Exploratory profiles of phenols, parabens, and per- and poly-fluoroalkyl substances among NHANES study participants in association with previous cancer diagnoses. J Expo Sci Environ Epidemiol. 2023;33(5):687–98. https://doi.org/10.1038/s41370-023-00601-6.
Grice MM, Alexander BH, Hoffbeck R, Kampa DM. Self-reported medical conditions in perfluorooctanesulfonyl fluoride manufacturing workers. J Occup Environ Med. 2007;49(7):722–9. https://doi.org/10.1097/JOM.0b013e3180582043.
Moon J, Mun Y. The association between per- and polyfluoroalkyl substances (PFASs) and brain, esophageal, melanomatous skin, prostate, and lung cancer using the 2003–2018 US National Health and nutrition examination survey (NHANES) datasets. Heliyon. 2024;10(2) https://doi.org/10.1016/j.heliyon.2024.e24337.
Frenoy P, Perduca V, Cano-Sancho G, Antignac JP, Severi G, Mancini FR. Application of two statistical approaches (Bayesian kernel machine regression and principal component regression) to assess breast cancer risk in association to exposure to mixtures of brominated flame retardants and per- and polyfluorinated alkylated substances in the E3N cohort. Environ Health. 2022;21(1):27. https://doi.org/10.1186/s12940-022-00840-4.
Lundin JI, Alexander BH, Olsen GW, Church TR. Ammonium perfluorooctanoate production and occupational mortality. Epidemiology. 2009;20(6):921–8. https://doi.org/10.1097/EDE.0b013e3181b5f395.
Gilliland FD, Mandel JS. Mortality among employees of a perfluorooctanoic acid production plant. J Occup Med. 1993;35(9):950–4. https://doi.org/10.1097/00043764-199309000-00020.
Girardi P, Merler E. A mortality study on male subjects exposed to polyfluoroalkyl acids with high internal dose of perfluorooctanoic acid. Environ Res. 2019;179(Pt A):108743. https://doi.org/10.1016/j.envres.2019.108743.
Rhee J, Barry KH, Huang WY, Sampson JN, Hofmann JN, Silverman DT, Calafat AM, Botelho JC, Kato K, Purdue MP, et al. A prospective nested case-control study of serum concentrations of per- and polyfluoroalkyl substances and aggressive prostate cancer risk. Environ Res. 2023;228:115718. https://doi.org/10.1016/j.envres.2023.115718.
Filippini T, Torres D, Lopes C, Carvalho C, Moreira P, Naska A, Kasdagli MI, Malavolti M, Orsini N, Vinceti M. Cadmium exposure and risk of breast cancer: a dose-response meta-analysis of cohort studies. Environ Int. 2020;142:105879. https://doi.org/10.1016/j.envint.2020.105879.
Cho E, Smith-Warner SA, Ritz J, van den Brandt PA, Colditz GA, Folsom AR, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. 2004;140(8):603–13. https://doi.org/10.7326/0003-4819-140-8-200404200-00007.
Godos J, Micek A, Brzostek T, Toledo E, Iacoviello L, Astrup A, Franco OH, Galvano F, Martinez-Gonzalez MA, Grosso G. Egg consumption and cardiovascular risk: a dose-response meta-analysis of prospective cohort studies. Eur J Nutr. 2021;60(4):1833–62. https://doi.org/10.1007/s00394-020-02345-7.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. https://doi.org/10.1002/sim.1186.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Halsne R, Tandberg JI, Lobert VH, Østby GC, Thoen E, Ropstad E, Verhaegen S. Effects of perfluorinated alkyl acids on cellular responses of MCF-10A mammary epithelial cells in monolayers and on acini formation in vitro. Toxicol Lett. 2016;259:95–107. https://doi.org/10.1016/j.toxlet.2016.08.004.
Wen LL, Lin CY, Chou HC, Chang CC, Lo HY, Juan SH. Perfluorooctanesulfonate mediates renal tubular cell apoptosis through PPARgamma inactivation. PLoS One. 2016;11(5):e0155190. https://doi.org/10.1371/journal.pone.0155190.
Di Nisio A, Rocca MS, Sabovic I, De Rocco PM, Corsini C, Guidolin D, Zanon C, Acquasaliente L, Carosso AR, De Toni L, et al. Perfluorooctanoic acid alters progesterone activity in human endometrial cells and induces reproductive alterations in young women. Chemosphere. 2020;242:125208. https://doi.org/10.1016/j.chemosphere.2019.125208.
Šabović I, Cosci I, De Toni L, Ferramosca A, Stornaiuolo M, Di Nisio A, Dall'Acqua S, Garolla A, Foresta C. Perfluoro-octanoic acid impairs sperm motility through the alteration of plasma membrane. J Endocrinol Investig. 2020;43(5):641–52. https://doi.org/10.1007/s40618-019-01152-0.
Pierozan P, Jerneren F, Karlsson O. Perfluorooctanoic acid (PFOA) exposure promotes proliferation, migration and invasion potential in human breast epithelial cells. Arch Toxicol. 2018;92(5):1729–39. https://doi.org/10.1007/s00204-018-2181-4.
Liu W, Irudayaraj J. Perfluorooctanoic acid (PFOA) exposure inhibits DNA methyltransferase activities and alters constitutive heterochromatin organization. Food Chem Toxicol. 2020;141:111358. https://doi.org/10.1016/j.fct.2020.111358.
Butenhoff JL, Chang SC, Olsen GW, Thomford PJ. Chronic dietary toxicity and carcinogenicity study with potassium perfluorooctanesulfonate in Sprague Dawley rats. Toxicology. 2012;293(1-3):1–15. https://doi.org/10.1016/j.tox.2012.01.003.
Pierozan P, Cattani D, Karlsson O. Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) induce epigenetic alterations and promote human breast cell carcinogenesis in vitro. Arch Toxicol. 2020;94(11):3893–906. https://doi.org/10.1007/s00204-020-02848-6.
Abdellatif AG, Préat V, Taper HS, Roberfroid M. The modulation of rat liver carcinogenesis by perfluorooctanoic acid, a peroxisome proliferator. Toxicol Appl Pharmacol. 1991;111(3):530–7. https://doi.org/10.1016/0041-008x(91)90257-f.
Abdellatif AG, Préat V, Vamecq J, Nilsson R, Roberfroid M. Peroxisome proliferation and modulation of rat liver carcinogenesis by 2,4-dichlorophenoxyacetic acid, 2,4,5-trichlorophenoxyacetic acid, perfluorooctanoic acid and nafenopin. Carcinogenesis. 1990;11(11):1899–902. https://doi.org/10.1093/carcin/11.11.1899.
Kodama S, Negishi M. Pregnane X receptor PXR activates the GADD45beta gene, eliciting the p38 MAPK signal and cell migration. J Biol Chem. 2011;286(5):3570–8. https://doi.org/10.1074/jbc.M110.179812.
Pierozan P, Kosnik M, Karlsson O. High-content analysis shows synergistic effects of low perfluorooctanoic acid (PFOS) and perfluorooctane sulfonic acid (PFOA) mixture concentrations on human breast epithelial cell carcinogenesis. Environ Int. 2023;172:107746. https://doi.org/10.1016/j.envint.2023.107746.
Olson CT, Andersen ME. The acute toxicity of perfluorooctanoic and perfluorodecanoic acids in male rats and effects on tissue fatty acids. Toxicol Appl Pharmacol. 1983;70(3):362–72. https://doi.org/10.1016/0041-008x(83)90154-0.
Vanden Heuvel JP. Perfluorodecanoic acid as a useful pharmacologic tool for the study of peroxisome proliferation. Gen Pharmacol. 1996;27(7):1123–9. https://doi.org/10.1016/0306-3623(95)00126-3.
Qin Y, Yuan X, Cui Z, Chen W, Xu S, Chen K, Wang F, Zheng F, Ni H, Shen HM, et al. Low dose PFDA induces DNA damage and DNA repair inhibition by promoting nuclear cGAS accumulation in ovarian epithelial cells. Ecotoxicol Environ Saf. 2023;265:115503. https://doi.org/10.1016/j.ecoenv.2023.115503.
Dominguez A, Salazar Z, Betancourt M, Ducolomb Y, Casas E, Fernandez F, Bahena I, Salomon A, Teteltitla M, Martinez R, et al. Effect of perfluorodecanoic acid on pig oocyte viability, intracellular calcium levels and gap junction intercellular communication during oocyte maturation in vitro. Toxicol in Vitro. 2019;58:224–9. https://doi.org/10.1016/j.tiv.2019.03.041.
Upham BL, Deocampo ND, Wurl B, Trosko JE. Inhibition of gap junctional intercellular communication by perfluorinated fatty acids is dependent on the chain length of the fluorinated tail. Int J Cancer. 1998;78(4):491–5.
Du Z, Cao YF, Li SN, Hu CM, Fu ZW, Huang CT, Sun XY, Liu YZ, Yang K, Fang ZZ. Inhibition of UDP-glucuronosyltransferases (UGTs) by phthalate monoesters. Chemosphere. 2018;197:7–13. https://doi.org/10.1016/j.chemosphere.2018.01.010.
Yang K, Fu ZW, Cao YF, Li SN, Du Z, Sun XY, Liu YZ, Yang K, Fang ZZ. New insights for risks of chlorophenols (CPs) exposure: inhibition of UDP-glucuronosyltransferases (UGTs). Chemosphere. 2018;206:9–16. https://doi.org/10.1016/j.chemosphere.2018.04.148.
Rogan EG, Badawi AF, Devanesan PD, Meza JL, Edney JA, West WW, Higginbotham SM, Cavalieri EL. Relative imbalances in estrogen metabolism and conjugation in breast tissue of women with carcinoma: potential biomarkers of susceptibility to cancer. Carcinogenesis. 2003;24(4):697–702. https://doi.org/10.1093/carcin/bgg004.
Liu YZ, Zhang ZP, Fu ZW, Yang K, Ding N, Hu LG, Fang ZZ, Zhuo X. Per- and polyfluoroalkyl substances display structure-dependent inhibition towards UDP-glucuronosyltransferases. Environ Pollut. 2019;254(Pt B):113093. https://doi.org/10.1016/j.envpol.2019.113093.
Tili E, Michaille JJ, Wernicke D, Alder H, Costinean S, Volinia S, Croce CM. Mutator activity induced by microRNA-155 (miR-155) links inflammation and cancer. Proc Natl Acad Sci USA. 2011;108(12):4908–13. https://doi.org/10.1073/pnas.1101795108.
Mishra A, Liu S, Sams GH, Curphey DP, Santhanam R, Rush LJ, Schaefer D, Falkenberg LG, Sullivan L, Jaroncyk L, et al. Aberrant overexpression of IL-15 initiates large granular lymphocyte leukemia through chromosomal instability and DNA hypermethylation. Cancer Cell. 2012;22(5):645–55. https://doi.org/10.1016/j.ccr.2012.09.009.
Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339(6117):286–91. https://doi.org/10.1126/science.1232227.
Bu P, Wang L, Chen KY, Srinivasan T, Murthy PK, Tung KL, Varanko AK, Chen HJ, Ai Y, King S, et al. A miR-34a-numb feedforward loop triggered by inflammation regulates asymmetric stem cell division in intestine and Colon Cancer. Cell Stem Cell. 2016;18(2):189–202. https://doi.org/10.1016/j.stem.2016.01.006.
Zhou X, Dong T, Fan Z, Peng Y, Zhou R, Wang X, Song N, Han M, Fan B, Jia J, et al. Perfluorodecanoic acid stimulates NLRP3 inflammasome assembly in gastric cells. Sci Rep. 2017;7:45468. https://doi.org/10.1038/srep45468.
Xu M, Zhang T, Lv C, Niu Q, Zong W, Tang J, Liu R. Perfluorodecanoic acid-induced oxidative stress and DNA damage investigated at the cellular and molecular levels. Ecotoxicol Environ Saf. 2019;185:109699. https://doi.org/10.1016/j.ecoenv.2019.109699.
Dong T, Peng Y, Zhong N, Liu F, Zhang H, Xu M, Liu R, Han M, Tian X, Jia J, et al. Perfluorodecanoic acid (PFDA) promotes gastric cell proliferation via sPLA2-IIA. Oncotarget. 2017;8(31):50911–20. https://doi.org/10.18632/oncotarget.17284.
Roque AT, Gambeloni RZ, Felitti S, Ribeiro ML, Santos JC. Inflammation-induced oxidative stress in breast cancer patients. Med Oncol. 2015;32(12):263. https://doi.org/10.1007/s12032-015-0709-5.
Cheng X, Klaassen CD. Critical role of PPAR-alpha in perfluorooctanoic acid- and perfluorodecanoic acid-induced downregulation of Oatp uptake transporters in mouse livers. Toxicol Sci. 2008;106(1):37–45. https://doi.org/10.1093/toxsci/kfn161.
Cimini A, Cristiano L, Bernardo A, Farioli-Vecchioli S, Stefanini S, Cerù MP. Presence and inducibility of peroxisomes in a human glioblastoma cell line. Biochim Biophys Acta. 2000;1474(3):397–409. https://doi.org/10.1016/s0304-4165(00)00036-2.
Kim SC, Hong JT, Jang SJ, Kang WS, Yoo HS, Yun YP. Formation of 8-oxodeoxyguanosine in liver DNA and hepatic injury by peroxisome proliferator clofibrate and perfluorodecanoic acid in rats. J Toxicol Sci. 1998;23(2):113–9. https://doi.org/10.2131/jts.23.2_113.
Borges T, Peterson RE, Pitot HC, Robertson LW, Glauert HP. Effect of the peroxisome proliferator perfluorodecanoic acid on the promotion of two-stage hepatocarcinogenesis in rats. Cancer Lett. 1993;72(1-2):111–20. https://doi.org/10.1016/0304-3835(93)90019-6.
Klaunig JE, Babich MA, Baetcke KP, Cook JC, Corton JC, David RM, DeLuca JG, Lai DY, McKee RH, Peters JM, et al. PPARalpha agonist-induced rodent tumors: modes of action and human relevance. Crit Rev Toxicol. 2003;33(6):655–780. https://doi.org/10.1080/713608372.
Funding
Wenxing Yang received funding from the National Natural Science Foundation of China (No. 32271178). The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This meta-analysis did not involve direct data collection from human or animal subjects and therefore did not require ethical approval from institutional or national research ethics committees.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary file 1
(DOCX 2046 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, J., Zhang, K., Shi, J. et al. Perfluoroalkyl and polyfluoroalkyl substances and Cancer risk: results from a does-response Meta-analysis. J Environ Health Sci Engineer (2024). https://doi.org/10.1007/s40201-024-00899-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40201-024-00899-w