Abstract
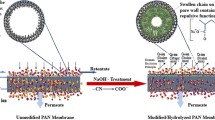
In this study, arsenate (As-V) removal using micellar enhanced ultrafiltration (MEUF) modified by cationic surfactants was studied by a dead-end polyacrylonitrile (PAN) membrane apparatus. The UF membrane has been produced by a phase inversion process. The prepared membrane was characterized and analyzed for morphology and membrane properties. The influence of operating parameters such as initial concentrations of As-V, surfactants, pH, membrane thickness, and co-existing anions on the removal of As-V, surfactant rejection, and permeate flux have been studied. The experimental results show that from the two different cationic surfactants used the CPC (cetyl-pyridinium chloride) efficiency (91.7%) was higher than that of HTAB (hexadecyltrimethyl-ammonium bromide) (83.7%). The highest As-V removal was 100%, and was achieved using initial feed concentrations of 100–1000 μg/L, at pH 7 with a membrane thickness of 150 μm in a dead-end filtration system. This efficiency for As-V removal was similar to that obtained using a cross-flow system. Nevertheless, this flux reduction was less than the reduction achieved in the dead-end filtration process. The PAN fabricated membrane in comparison to the RO and NF processes selectively removed the arsenic and the anions, in the water taken from the well, and had no substantial effect on the cations.















Similar content being viewed by others
References
Liu X, Zhang W, Hu Y, Hu E, Xie X, Wang L, et al. Arsenic pollution of agricultural soils by concentrated animal feeding operations (CAFOs). Chemosphere. 2015;119:273–81.
Jais FM, Ibrahim S, Yoon Y, Jang M. Enhanced arsenate removal by lanthanum and nano–magnetite composite incorporated palm shell waste–based activated carbon. Sep Purif Technol. 2016;169:93–102.
Zazouli MA, Bandpei AM, Maleki A, Saberian M, Izanloo H. Determination of cadmium and lead contents in black tea and tea liquor from Iran. Asian J Chem. 2010;22(2):1387.
Lien H-L, Wilkin RT. High-level arsenite removal from groundwater by zero-valent iron. Chemosphere. 2005;59(3):377–86.
Rezaee R, Nasseri S, Mahvi AH, Nabizadeh R, Mousavi SA, Rashidi A, et al. Fabrication and characterization of a polysulfone-graphene oxide nanocomposite membrane for arsenate rejection from water. J Environ Health Sci Eng. 2015;13(1):61.
Agency for Toxic Substances and Diesease Registry (ATSDR), ToxFAQs for arsenic. (2001).
Water, U.S.E.P.A.O.o.: 2004 Edition of the drinking water standards and health advisories. United States Environmental Protection Agency, Office of Water, (2004).
Organization, W.H.: Guidelines for drinking-water quality: incorporating first and second addenda to third edition, Vol. 1, Recommendations. In. Geneva: WHO Press, (2008).
Ebrahimi R, Maleki A, Shahmoradi B, Daraei H, Mahvi AH, Barati AH, et al. Elimination of arsenic contamination from water using chemically modified wheat straw. Desalin Water Treat. 2013;51(10–12):2306–16.
Jiang Y, Zeng X, Fan X, Chao S, Zhu M, Cao H. Levels of arsenic pollution in daily foodstuffs and soils and its associated human health risk in a town in Jiangsu Province, China. Ecotoxicol Environ Saf. 2015;122:198–204.
McArthur J, Ghosal U, Sikdar P, Ball J. Arsenic in groundwater: the deep late pleistocene aquifers of the Western Bengal Basin. Environ Sci Technol. 2016;50(7):3469–76.
Jebelli MA, Maleki A, Amoozegar MA, Kalantar E, Shahmoradi B, Gharibi F. Isolation and identification of indigenous prokaryotic bacteria from arsenic-contaminated water resources and their impact on arsenic transformation. Ecotoxicol Environ Saf. 2017;140:170–6.
Barati A, Maleki A, Alasvand M. Multi-trace elements level in drinking water and the prevalence of multi-chronic arsenical poisoning in residents in the west area of Iran. Sci Total Environ. 2010;408(7):1523–9.
Mohanty D. Conventional as well as emerging arsenic removal technologies—a critical review. Water Air Soil Pollut. 2017;228(10):381.
Gecol H, Ergican E, Fuchs A. Molecular level separation of arsenic (V) from water using cationic surfactant micelles and ultrafiltration membrane. J Membr Sci. 2004;241(1):105–19.
Lakshmipathiraj P, Prabhakar S, Raju GB. Studies on the electrochemical decontamination of wastewater containing arsenic. Sep Purif Technol. 2010;73(2):114–21.
Meng C, Mao Q, Luo L, Zhang J, Wei J, Yang Y, et al. Performance and mechanism of as (III) removal from water using Fe-Al bimetallic material. Sep Purif Technol. 2017.
Jebeli MA, Maleki A, Amoozegar MA, Kalantar E, Izanloo H, Gharibi F. Bacillus flexus strain as-12, a new arsenic transformer bacterium isolated from contaminated water resources. Chemosphere. 2017;169:636–41.
Chen M, Shafer-Peltier K, Randtke SJ, Peltier E. Modeling arsenic (V) removal from water by micellar enhanced ultrafiltration in the presence of competing anions. Chemosphere. 2018;213:285–94.
Zheng Y, Wang A. Removal of heavy metals using polyvinyl alcohol semi-IPN poly (acrylic acid)/tourmaline composite optimized with response surface methodology. Chem Eng J. 2010;162(1):186–93.
Cui J, Jing C, Che D, Zhang J, Duan S. Groundwater arsenic removal by coagulation using ferric (III) sulfate and polyferric sulfate: a comparative and mechanistic study. J Environ Sci. 2015;32:42–53.
Hong J, Zhu Z, Lu H, Qiu Y. Synthesis and arsenic adsorption performances of ferric-based layered double hydroxide with α-alanine intercalation. Chem Eng J. 2014;252:267–74.
Misra R, Jain S, Khatri P. Iminodiacetic acid functionalized cation exchange resin for adsorptive removal of Cr (VI), cd (II), Ni (II) and Pb (II) from their aqueous solutions. J Hazard Mater. 2011;185(2):1508–12.
Dominguez-Ramos A, Chavan K, García Vn, Jimeno G, Albo J, Marathe KV, et al. Arsenic removal from natural waters by adsorption or ion exchange: an environmental sustainability assessment. Ind Eng Chem Res. 2014;53(49):18920–7.
Molinari R, Argurio P. Arsenic removal from water by coupling photocatalysis and complexation-ultrafiltration processes: a preliminary study. Water Res. 2017;109:327–36.
Mondal P, Tran ATK, Van der Bruggen B. Removal of as (V) from simulated groundwater using forward osmosis: effect of competing and coexisting solutes. Desalination. 2014;348:33–8.
Xiao S, Ma H, Shen M, Wang S, Huang Q, Shi X. Excellent copper (II) removal using zero-valent iron nanoparticle-immobilized hybrid electrospun polymer nanofibrous mats. Colloids Surf A Physicochem Eng Asp. 2011;381(1):48–54.
Krishnamoorthy R, Sagadevan V. Polyethylene glycol and iron oxide nanoparticles blended polyethersulfone ultrafiltration membrane for enhanced performance in dye removal studies. E-Polymers. 2015;15(3):151–9.
Wang X, Fang D, Yoon K, Hsiao BS, Chu B. High performance ultrafiltration composite membranes based on poly (vinyl alcohol) hydrogel coating on crosslinked nanofibrous poly (vinyl alcohol) scaffold. J Membr Sci. 2006;278(1):261–8.
Samper E, Rodríguez M, De la Rubia M, Prats D. Removal of metal ions at low concentration by micellar-enhanced ultrafiltration (MEUF) using sodium dodecyl sulfate (SDS) and linear alkylbenzene sulfonate (LAS). Sep Purif Technol. 2009;65(3):337–42.
Juang R-S, Lin S-H, Peng L-C. Flux decline analysis in micellar-enhanced ultrafiltration of synthetic waste solutions for metal removal. Chem Eng J. 2010;161(1–2):19–26.
Huang J, Yuan F, Zeng G, Li X, Gu Y, Shi L, et al. Influence of pH on heavy metal speciation and removal from wastewater using micellar-enhanced ultrafiltration. Chemosphere. 2017;173:199–206.
Rahmanian B, Pakizeh M, Maskooki A. Micellar-enhanced ultrafiltration of zinc in synthetic wastewater using spiral-wound membrane. J Hazard Mater. 2010;184(1–3):261–7.
Iqbal J, Kim H-J, Yang J-S, Baek K, Yang J-W. Removal of arsenic from groundwater by micellar-enhanced ultrafiltration (MEUF). Chemosphere. 2007;66(5):970–6.
Dunn RO Jr, Scamehorn JF, Christian SD. Use of micellar-enhanced ultrafiltration to remove dissolved organics from aqueous streams. Sep Sci Technol. 1985;20(4):257–84.
De, S., Mondal, S.: Micellar enhanced ultrafiltration: Fundamentals & Applications. CRC Press, (2012).
Purkait M, DasGupta S, De S. Micellar enhanced ultrafiltration of eosin dye using hexadecyl pyridinium chloride. J Hazard Mater. 2006;136(3):972–7.
Camarillo R, Asencio I, Rincón J. Micellar enhanced ultrafiltration for phosphorus removal in domestic wastewater. Desalin Water Treat. 2009;6(1–3):211–6.
Baek K, Kim B-K, Yang J-W. Application of micellar enhanced ultrafiltration for nutrients removal. Desalination. 2003;156(1–3):137–44.
Jana DK, Roy K, Dey S. Comparative assessment on lead removal using micellar-enhanced ultrafiltration (MEUF) based on a type-2 fuzzy logic and response surface methodology. Sep Purif Technol. 2018;207:28–41.
Huang J, Li H, Zeng G, Shi L, Gu Y, Shi Y, et al. Removal of cd (II) by MEUF-FF with anionic-nonionic mixture at low concentration. Sep Purif Technol. 2018.
Grzegorzek M, Majewska-Nowak K. The use of micellar-enhanced ultrafiltration (MEUF) for fluoride removal from aqueous solutions. Sep Purif Technol. 2018;195:1–11.
Geanta RM, Ruiz MO, Escudero I. Micellar-enhanced ultrafiltration for the recovery of lactic acid and citric acid from beet molasses with sodium dodecyl sulphate. J Membr Sci. 2013;430:11–23.
Acero JL, Benitez FJ, Real FJ, Teva F. Removal of emerging contaminants from secondary effluents by micellar-enhanced ultrafiltration. Sep Purif Technol. 2017;181:123–31.
Huang J, Peng L, Zeng G, Li X, Zhao Y, Liu L, et al. Evaluation of micellar enhanced ultrafiltration for removing methylene blue and cadmium ion simultaneously with mixed surfactants. Sep Purif Technol. 2014;125:83–9.
Tanhaei B, Chenar MP, Saghatoleslami N, Hesampour M, Laakso T, Kallioinen M, et al. Simultaneous removal of aniline and nickel from water by micellar-enhanced ultrafiltration with different molecular weight cut-off membranes. Sep Purif Technol. 2014;124:26–35.
Li Q, Jensen JO, Savinell RF, Bjerrum NJ. High temperature proton exchange membranes based on polybenzimidazoles for fuel cells. Prog Polym Sci. 2009;34(5):449–77.
Park JS, Lee SH, Han TH, Kim SO. Hierarchically ordered polymer films by templated organization of aqueous droplets. Adv Funct Mater. 2007;17(14):2315–20.
Khamforoush M, Pirouzram O, Hatami T. The evaluation of thin film composite membrane composed of an electrospun polyacrylonitrile nanofibrous mid-layer for separating oil–water mixture. Desalination. 2015;359:14–21.
Jana S, Saikia A, Purkait M, Mohanty K. Chitosan based ceramic ultrafiltration membrane: preparation, characterization and application to remove hg (II) and as (III) using polymer enhanced ultrafiltration. Chem Eng J. 2011;170(1):209–19.
Baek K, Yang T-W. Competitive bind of anionic metals with cetylpyridinium chloride micelle in micellar-enhanced ultrafiltration. Desalination. 2004;167:101–10.
Kim B-K, Baek K, Yang J-W. Simultaneous removal of nitrate and phosphate using cross-flow micellar-enhanced ultrafiltration (MEUF). Water Sci Technol. 2004;50(6):227.
Beolchini F, Pagnanelli F, De Michelis I, Vegliò F. Micellar enhanced ultrafiltration for arsenic (V) removal: effect of main operating conditions and dynamic modelling. Environ Sci Technol. 2006;40(8):2746–52.
Malaisamy R, Berry D, Holder D, Raskin L, Lepak L, Jones KL. Development of reactive thin film polymer brush membranes to prevent biofouling. J Membr Sci. 2010;350(1–2):361–70.
Beolchini F, Pagnanelli F, De Michelis I, Veglio F. Treatment of concentrated arsenic (V) solutions by micellar enhanced ultrafiltration with high molecular weight cut-off membrane. J Hazard Mater. 2007;148(1–2):116–21.
Ergican E, Gecol H, Fuchs A. The effect of co-occurring inorganic solutes on the removal of arsenic (V) from water using cationic surfactant micelles and an ultrafiltration membrane. Desalination. 2005;181(1–3):9–26.
Morel G, Ouazzani N, Graciaa A, Lachaise J. Surfactant modified ultrafiltration for nitrate ion removal. J Membr Sci. 1997;134(1):47–57.
Juang R-S, Xu Y-Y, Chen C-L. Separation and removal of metal ions from dilute solutions using micellar-enhanced ultrafiltration. J Membr Sci. 2003;218(1):257–67.
Tanhaei B, Chenar MP, Saghatoleslami N, Hesampour M, Kallioinen M, Sillanpää M, et al. Removal of nickel ions from aqueous solution by micellar-enhanced ultrafiltration, using mixed anionic–non-ionic surfactants. Sep Purif Technol. 2014;138:169–76.
Baek K, Lee H-H, Yang J-W. Micellar-enhanced ultrafiltration for simultaneous removal of ferricyanide and nitrate. Desalination. 2003;158(1–3):157–66.
Rosen, M.J., Kunjappu, J.T.: Surfactants and interfacial phenomena. John Wiley & Sons, (2012).
Purkait M, DasGupta S, De S. Removal of dye from wastewater using micellar-enhanced ultrafiltration and recovery of surfactant. Sep Purif Technol. 2004;37(1):81–92.
Olcay AN, Polat M, Polat H. Ancillary effects of surfactants on filtration of low molecular weight contaminants through cellulose nitrate membrane filters. Colloids Surf A Physicochem Eng Asp. 2016;492:199–206.
Liu Y, Wang R, Ma H, Hsiao BS, Chu B. High-flux microfiltration filters based on electrospun polyvinylalcohol nanofibrous membranes. Polymer. 2013;54(2):548–56.
Wimalawansa SJ. Purification of contaminated water with reverse osmosis: effective solution of providing clean water for human needs in developing countries. J Emerg Technol Adv Eng. 2013;3(12):75–89.
Acknowledgements
This manuscript is extracted from the Ph.D. thesis of the first author and approved by the Environmental Health Research Center and funded by the Kurdistan University of Medical Sciences (IR.MUK.REC.1394/57). The authors offer their thanks to the sponsors of the project. We also thank Prof. Mohammad Ali Zazouli for the scientific comments in editing the article.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bahmani, P., Maleki, A., Rezaee, R. et al. Arsenate removal from aqueous solutions using micellar-enhanced ultrafiltration. J Environ Health Sci Engineer 17, 115–127 (2019). https://doi.org/10.1007/s40201-018-00332-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-018-00332-z