Abstract
Background
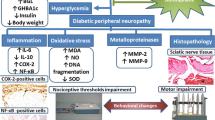
Neuropathy as a common complication of hyperglycemia in diabetic patients is probably caused by metabolic and structural changes in extracellular matrix (ECM) of peripheral nerves. This study was designed to evaluate the effects of benfotiamine (BT) on the structural, biological and mechanical characteristics of rat sciatic nerve in hyperglycemic condition.
Materials and methods
Forty eight adult male Wistar rats were assigned to 6 groups (n = 8): control (healthy rats with no treatment; C), positive control (healthy rats received BT treatment; B), negative control groups 1&2 (hyperglycemic rats kept for 4 and/or 8 weeks; 4WD and 8WD, respectively) and experimental groups 1&2 (hyperglycemic rats treated by daily oral gavage of 100 mg kg− 1 body weight BT for 4 and/or 8 weeks; 4WD + BT and 8WD + BT, respectively). Hyperglycemia was induced by a single intraperitoneal injection of of streptozotocin (55 mg kg− 1 body weight). After a period of experimental period (4 and/or 8 weeks) rats were sacrificed and from each two segments (1 cm length) of left sciatic nerve were sampled. These samples were prepared for histological examinations (light and electron microscopy), collagen IV immunohistochemistry and strength tensile test.
Results
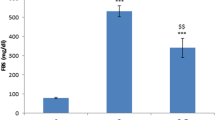
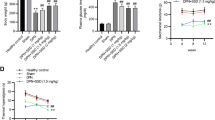
In comparison to control groups, in 4WD and 8WD groups the amount of type IV collagen was increased, the structure of myelin sheath and nerve fibers were extensively altered and the tensile strength was significantly decreased (p < 0.05) while in 4WD + BT and 8WD + BT groups these abnormalities were attenuated.
Conclusions
It seems that BT treatment may rescue the sciatic nerve from the hyperglycemic-induced ECM structural abnormality. This beneficial advantage of BT is likely exerted through the modification of glucose metabolism pathways.









Similar content being viewed by others
References
Rassouli MB, Ghayour MB, Ghayour N. Microvascular complications of diabetes. J Biol Sci. 2010;10:411–23.
Diabéticas Periféricas N, et al. Diabetic peripheral neuropathies: a morphometric overview. Int J Morphol. 2010;28(1):51–64.
Vinik AI, Nevoret M, Casellini C, Parson H. Diabetic neuropathy. Endocrinol Metab Clin North Am. 2013;42(4):747-87 (Elsevier Inc).
Bruschi LKM, et al. Diabetes mellitus and diabetic peripheral neuropathy. Open J Endocr Metab Dis. 2017;07(01):12–21.
Yagihashi S, Mizukami H, Sugimoto K. Mechanism of diabetic neuropathy: Where are we now and where to go? J Diabetes Investig. 2011;2(1):18–32.
Power SE, et al. The potential role of thiamine (vitamin B1) in diabetic complications. ” Nutr J. 2013;12(3):148.
Kawano T. A current overview of diabetic neuropathy – mechanisms, symptoms, diagnosis, and treatment. Peripher Neuropathy. 2014:89–105.
Luo X, Wu J, Jing S, Yan L-J. Hyperglycemic stress and carbon stress in diabetic glucotoxicity. Aging Dis. 2016;7(1):90.
Sadowska-Bartosz I, Bartosz G. Prevention of protein glycation by natural compounds. Molecules. 2015;20(2):3309–34.
Schalkwijk CG, Stehouwer CDA, van Hinsbergh VWM. Fructose-mediated non-enzymatic glycation: Sweet coupling or bad modification. Diabetes Metab Res Rev. 2004;20(5):369–82.
Duran-jimenez B, et al. Advanced glycation end products in extracellular matrix proteins contribute to the failure of sensory nerve regeneration in diabetes. Sens Neur. 2009;58(December):2893–903.
Oztürk G, Sekeroğlu MR, Erdoğan E, Oztürk M. The effect of non-enzymatic glycation of extracellular matrix proteins on axonal regeneration in vitro. ” Acta Neuropathol. 2006;112(5):627–32.
Tan KCB, et al. Advanced glycation end products and diabetic complications: a general overview. Hormones (Athens). 2005;4(1):28–37.
Kumar Pasupulati A, Chitra PS, Reddy GB. Advanced glycation end products mediated cellular and molecular events in the pathology of diabetic nephropathy. Biomol Concepts. 2016;7(5–6):293–299.
Hill RE, Williams PE. A quantitative analysis of perineurial cell basement membrane collagen IV, laminin and fibronectin in diabetic and non-diabetic human sural nerve. J Anat. 2002;201(2):185–92.
Luong. The impact of thiamine treatment in the diabetes mellitus. J Clin Med Res. 2012;4(3):153–60.
Greb A, Bitsch R. Comparative bioavailability of various thiamine derivatives after oral administration. Int J Clin Pharmacol Ther. 1998;36(4):216–21.
Brownlee M. Lilly lecture 1993: Glycation and diabetic complications. Diabetes. 1994;43(6):836–41.
Stracke H, Gaus W, Achenbach U, Federlin K, Bretzel RG. Benfotiamine in diabetic polyneuropathy (BENDIP): Results of a randomised, double blind, placebo-controlled clinical study. Exp Clin Endocrinol Diabetes. 2008;116(10):600–5.
Alam SS. Effect of high dose thiamine therapy on risk factors in type 2 diabetics. J Diabetes Metab. 2012;03(10).
Varadi G, Zhu Z, Carter SG. Efficient transdermal delivery of benfotiamine in an animal model. Admet Dmpk. 2015;2(4):272–81.
Karamoysoyli E, Burnand RC, Tomlinson DR, Gardiner NJ. Neuritin mediates nerve growth factor – induced axonal. Diabetes. 2008;57(January):181–9.
Volvert ML, et al. Benfotiamine, a synthetic S-acyl thiamine derivative, has different mechanisms of action and a different pharmacological profile than lipid-soluble thiamine disulfide derivatives. BMC Pharmacol. 2008;8:1–11.
Mohammadi R, Saadati A. Influence of insulin-like growth factor I on nerve regeneration using allografts: A scia[1] R. Mohammadi, Saadati A, ‘Influence of insulin-like growth factor I on nerve regeneration using allografts: A sciatic nerve model’, J. Craniofac. Surg., vol. 2. J Craniofac Surg. 2014;25(4):1510–4.
Kumar GL, Kiernan J. Special stains and H & E Second Edition Education Guide | Special Stains and H & E. Dako North Am Carpint Calif, p. 158, 2010.
Aldaghi MR, Jalali M, Nikravesh MR, Fazel A. Research opinions in animal & veterinary sciences effect of α -lipoic acid on expression of collagen IV of the sciatic nerve of diabetic rats, pp. 576–582, 2008.
Tennakoon JB. Methods in molecular biology, no. November 2013. 2014.
Sondell M, Lundborg G, Kanje M. Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res. 1998;795:1–2.
Borschel GH, Kia KF, Kuzon WM, Dennis RG. Mechanical properties of acellular peripheral nerve. J Surg Res. 2003;114(2):133–9.
Exposito JY, Valcourt U, Cluzel C, Lethias C. The fibrillar collagen family. Int J Mol Sci. 2010;11(2):407–26.
Laurie GW, Leblond CP, Martin GR. Localization of type IV collagen, laminin, heparin sulphate proteoglycan, and fibronectin to the basal lamina of basement membranes. J Cell Biol. 1984;26(October):107–16.
Eble JA, Golbik R, Mann K, Kühn K. The alpha 1 beta 1 integrin recognition site of the basement membrane collagen molecule [alpha 1(IV)]2 alpha 2(IV). EMBO J. 1993;12(12):4795–802.
Fox JW, et al. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. ” EMBO J. 1991;10(11):3137–46.
King RHM, Llewelyn JG, Thomas PK, Gilbey SG, Watkins PJ. Diabetic neuropathy: abnormalities of schwann cell and perineurial basal laminae. implications for diabetic vasculopathy. Neuropathol Appl Neurobiol. 1989;15(4):339–55.
Johnson PC, Doll SC. Dermal nerves in human diabetic subjects. Diabetes. 1984;33(3):244–50.
Johnson PC. Thickening of the human dorsal root ganglion perineural cell basement membrane in diabetis mellitus. Muscle Nerve. 1983;6(October):561–5.
Bradley JL, King RH, Muddle JR, Thomas PK. The extracellular matrix of peripheral nerve in diabetic polyneuropathy. ” Acta Neuropathol. 2000;99(5):539–46.
Ma ST, Liu DL, Deng JJ, Peng YJ. Protective effect of mulberry flavonoids on sciatic nerve in alloxan–induced diabetic rats. Braz J Pharm Sci. 2014;50(4):765–72.
Gao S, et al. Comparison of morphology and biocompatibility of acellular nerve scaffolds processed by different chemical methods. J Mater Sci Mater Med. 2014;25(5):1283–91.
Žilić L. Development of acellular porcine peripheral nerves, no. October, 2016.
Luo ZJ, King RHM, Lewin J, Thomas PK. Effects of nonenzymatic glycosylation of extracellular matrix components on cell survival and sensory neurite extension in cell culture. J Neurol. 2002;249(4):424–31.
Berrone E, Beltramo E, Solimine C, Ape AU, Porta M. Regulation of intracellular glucose and polyol pathway by thiamine and benfotiamine in vascular cells cultured in high glucose. J Biol Chem. 2006;281(14):9307–13.
Beltramo E, Berrone E, Tarallo S, Porta M. Effects of thiamine and benfotiamine on intracellular glucose metabolism and relevance in the prevention of diabetic complications. Acta Diabetol. 2008;45(3):131–41.
Jamali R, Mohseni S. Differential neuropathies in hyperglycemic and hypoglycemic diabetic rats. J Neuropathol Exp Neurol. 2006;65(12):1118–25.
Behse F, Buchthal F. Nerve biopsy and conduction studies in diabetic neuropathy. pp. 1072–1082, 1977.
Chalothorn D, et al. Differential cardiovascular regulatory activities of the alpha1B- and alpha1D-adrenoceptor subtypes. J Pharmacol Exp Ther. 2003;305(3):1045–53.
Goh S-Y, Cooper ME. The role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab. 2008;93(4):1143–52.
Hill RE, Williams PE. Perineurial cell basement membrane thickening and myelinated nerve fibre loss in diabetic and nondiabetic peripheral nerve. J Neurol Sci. 2004;217(2):157–63.
Layton BE, et al. Differences between collagen morphologies, properties and distribution in diabetic and normal biobreeding and Sprague-Dawley rat sciatic nerves. J Biomech. 2004;37(6):879–88.
Terai N, Spoerl E, Haustein M, Hornykewycz K, Haentzschel J, Pillunat LE. 10.1159/000331990. Ophthalmic Res. 2012;47(4):189–94.
Ishibashi F, Taniguchi M, Kojima R, Kawasaki A, Kosaka A, Uetake H. Elasticity of the tibial nerve assessed by sonoelastography was reduced before the development of neuropathy and further deterioration associated with the severity of neuropathy in patients with type 2 diabetes. J Diabetes Investig. 2016;7(3):404–12.
Zhu Z, Varadi G, Carter SG. Pharmacokinetics of the transdermal delivery of benfotiamine. Acta Diabetol. 2016;53(2):317–22.
Acknowledgements
This work was supported by a grant (No. 41853) from Ferdowsi University of Mashhad, which is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
In clinic, benfotiamine is prescribed for the treatment of diabetic neuropathy. In the present research the preventive effect of BT on hyperglycemic-induced structural abnormalities of sciatic nerve are highlighted.
Rights and permissions
About this article
Cite this article
Vafadar Ghasemi, L., Behnam Rassouli, M., Matin, M.M. et al. Benfotiamine reduced collagen IV contents of sciatic nerve in hyperglycemic rats. J Diabetes Metab Disord 20, 21–30 (2021). https://doi.org/10.1007/s40200-020-00666-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-020-00666-2