Abstract
Purpose
Diabetes mellitus is a prevalent metabolic disorder that entails numerous complications in various organs. In current era, different types of diseases are being treated by the applications of herbs. The present study is aimed at investigating the anti-inflammatory and antioxidant effects of the Rubus fruticosus hydroethanolic extracts (RFHE) in the streptozotocin (STZ)-induced diabetic rats.
Methods
At this experimental research, male Wistar rats with the weight of 220 ± 20 g, were categorized randomly into five groups of vehicles as control, STZ (60 mg kg− 1 of body weight, intraperitoneally (i.p.)) and RFHE (50, 100 and 200 mg kg− 1, i.p.). In the last stage (end of week 4) of the experiment, after being euthanized, the blood samples of the rats were collected for measuring malondialdehyde (MDA), glutathione (GSH), total antioxidant status (TAS) as well as inflammatory markers like tumor necrosis factor (TNF)-α, interleukin (IL)-6 and C-reactive protein (CRP).
Results
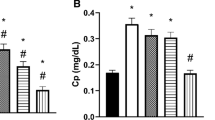
Data from this study was revealed that diabetes causes oxidative damage and consequently the serum level of inflammatory markers rises. RFHE was shown to be significantly correlated with lowering the level of MDA, TNF-α, IL-6 and CRP of diabetic rats. Moreover, RFHE significantly elevated the GSH and TAS serum levels in diabetic rats when compared with STZ group.
Conclusions
RFHE might have anti-diabetic properties; this outcome may be mediated by high antioxidant and anti-inflammatory effects.


Similar content being viewed by others
References
Taheri E, Saedisomeolia A, Djalali M, Qorbani M, Madani Civi M. The relationship between serum 25-hydroxy vitamin D concentration and obesity in type 2 diabetic patients and healthy subjects. J Diabetes Metab Disord. 2012;11(1):16-. https://doi.org/10.1186/2251-6581-11-16.
Larejani B, Zahedi F. Epidemiology of diabetes mellitus in Iran. Iran J Diabetes Lipid Disord. 2001;1(1):1–8.
Meraci M, Feizi A, Bagher Nejad M. Investigating the prevalence of high blood pressure, type 2 diabetes mellitus and related risk factors according to a large general study in Isfahan- using multivariate logistic regression model. Health Syst Res. 2012;8(2).
Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabetic Med. 1997;14(Suppl 5):1–85.
Daneman D. Type 1 diabetes. Lancet. 2006;367(9513):847–58. doi:https://doi.org/10.1016/S0140-6736(06)68341-4.
Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50(5):567–75. https://doi.org/10.1016/j.freeradbiomed.2010.12.006.
King GL. The role of inflammatory cytokines in diabetes and its complications. J Periodontol. 2008;79(8 Suppl):1527–34. doi:https://doi.org/10.1902/jop.2008.080246.
Mohajeri D, Doustar Y, Rezaei A, Mesgari Abbasi M. Hepatoprotective Effect of ethanolic extract of Crocus sativus L. (saffron) stigma in comparison with silymarin against rifampin induced hepatotoxicity in rats. Zahedan J Res Med Sci. 2011;12:53–9.
Zia-Ul-Haq M, Riaz M, De Feo V, Jaafar HZ, Moga M. Rubus fruticosus L.: constituents, biological activities and health related uses. Molecules. 2014;19(8):10998–1029. doi:https://doi.org/10.3390/molecules190810998.
Monforte MT, Smeriglio A, Germanò MP, Pergolizzi S, Circosta C, Galati EM. Evaluation of antioxidant, antiinflammatory, and gastroprotective properties of Rubus fruticosus L. fruit juice. Phytother Res. 2018;32(7):1404–14. doi:https://doi.org/10.1002/ptr.6078.
Gomar A, Hosseini A, Mirazi N. Preventive effect of Rubus fruticosus on learning and memory impairment in an experimental model of diabetic neuropathy in male rats. Pharma Nutr. 2014;2(4):155–60.
Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8.
Budin SB, Othman F, Louis SR, Bakar MA, Das S, Mohamed J. The effects of palm oil tocotrienol-rich fraction supplementation on biochemical parameters, oxidative stress and the vascular wall of streptozotocin-induced diabetic rats. Clinics. 2009;64(3):235–44. doi:https://doi.org/10.1590/s1807-59322009000300015.
Biswas D, Banerjee M, Sen G, Das JK, Banerjee A, Sau TJ, et al. Mechanism of erythrocyte death in human population exposed to arsenic through drinking water. Toxicol Appl Pharmcol. 2008;230(1):57–66. doi:https://doi.org/10.1016/j.taap.2008.02.003.
Yagihashi S, Yamagishi S-I, Wada R. Pathology and pathogenetic mechanisms of diabetic neuropathy: correlation with clinical signs and symptoms. Diabetes Res Clin Pract. 2007;77(Suppl 1):184-S9. doi:https://doi.org/10.1016/j.diabres.2007.01.054.
Calcutt NA, Freshwater JD, Mizisin AP. Prevention of sensory disorders in diabetic Sprague-Dawley rats by aldose reductase inhibition or treatment with ciliary neurotrophic factor. Diabetologia. 2004;47(4):718–24. doi:https://doi.org/10.1007/s00125-004-1354-2.
Boyer F, Vidot JB, Dubourg AG, Rondeau P, Essop MF, Bourdon E. Oxidative stress and adipocyte biology: focus on the role of AGEs. Oxid Med Cell Longev. 2015;2015:534873-. doi:https://doi.org/10.1155/2015/534873.
Pasaoglu H, Sancak B, Bukan N. Lipid peroxidation and resistance to oxidation in patients with type 2 diabetes mellitus. Tohoku J Exp Med. 2004;203(3):211–8. doi:https://doi.org/10.1620/tjem.203.211.
Rehman K, Akash MSH. Mechanism of generation of oxidative stress and pathophysiology of type 2 diabetes mellitus: How are they interlinked? J Cell Biochem. 2017;118(11):3577–85. https://doi.org/10.1002/jcb.26097.
Domingueti CP, Dusse LMSA, Carvalho MdG, de Sousa LP, Gomes KB, Fernandes AP. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complicat. 2016;30(4):738–45. https://doi.org/10.1016/j.jdiacomp.2015.12.018.
Ma H, Johnson SL, Liu W, DaSilva NA, Meschwitz S, Dain JA, et al. Evaluation of polyphenol anthocyanin-enriched extracts of blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry for free radical scavenging, reactive carbonyl species trapping, anti-glycation, anti-β-amyloid aggregation, and microglial neuroprotective effects. Int J Mol Sci. 2018;19(2):461. https://doi.org/10.3390/ijms19020461.
Caidan R, Cairang L, Pengcuo J, Tong L. Comparison of compounds of three Rubus species and their antioxidant activity. Drug Discov Ther. 2015;9(6):391–6. doi:https://doi.org/10.5582/ddt.2015.01179.
Abdu D, Majeed SNJJAC. Identification of antioxidant compounds in red raspberry (Rubus idaeus) fruit in Kurdistan region (North Iraq). 2012;2(3):6–10.
Abdel-Rahman RF, Soliman GA, Saeedan AS, Ogaly HA, Abd-Elsalam RM, Alqasoumi SI, et al. Molecular and biochemical monitoring of the possible herb-drug interaction between Momordica charantia extract and glibenclamide in diabetic rats. Saudi Pharm J. 2019;27(6):803–16. doi:https://doi.org/10.1016/j.jsps.2019.05.002.
Jean-Gilles D, Li L, Ma H, Yuan T, Chichester CO III, Seeram NP. Anti-inflammatory effects of polyphenolic-enriched red raspberry extract in an antigen-induced arthritis rat model. J Agric Food Chem. 2012;60(23):5755–62. doi:https://doi.org/10.1021/jf203456w.
Sangiovanni E, Vrhovsek U, Rossoni G, Colombo E, Brunelli C, Brembati L, et al. Ellagitannins from Rubus berries for the control of gastric inflammation: in vitro and in vivo studies. PLoS One. 2013;8(8):e71762. doi:https://doi.org/10.1371/journal.pone.0071762.
Funding
The authors have declared that there were no funding received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest disclosure
The authors wish to declare that there were no competing interests.
Research involving human participants and/or animals
All the ethical considerations of working with the animals were accounted for (Guide for the Care and Use of Laboratory Animals, 8th edition, National Academies Press). Furthermore, the Ethics Committee of Bu-Ali Sina University, confirmed the validity of this project (IR.BASU.REC.1397.036).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mirazi, N., Hosseini, A. Attenuating properties of Rubus fruticosus L. on oxidative damage and inflammatory response following streptozotocin-induced diabetes in the male Wistar rats. J Diabetes Metab Disord 19, 1311–1316 (2020). https://doi.org/10.1007/s40200-020-00649-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-020-00649-3