Abstract
Aims
This study aimed to investigate the carbohydrate and lipid dynamics, associated inflammation markers and the effectiveness of a grape-derived stilbene concentrate (GDSC) treatment in experimental metabolic syndrome (MetS).
Methods
The study was carried out on 40 male 12-weeks of age Wistar rats. The MetS was induced using the fructose model (feeding with 60%-solid fructose diet for 24 weeks). Rats with induced MetS were treated with polyphenolic GDSC, which was obtained by water-alcohol extraction of Vitis vinifera grapevine (Ressfood LLC, Russia).
Results
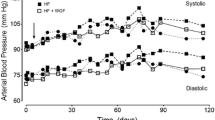
The experimentally induced MetS development leads to classic MetS signs, including abdominal obesity, hyperglycemia, high lipid levels and heart damage. The expression of glucose transporter type 4 (GLUT4) and peroxisome proliferator-activated receptor-γ (PPAR-γ) had greater dynamics than biochemical measurements. The development of the associated inflammatory reactions was confirmed by the increased level of Toll-like receptor type 4 (TLR4) and C-reactive protein (CRP) compared to control levels. The use of the GDSC had positive dynamics in carbohydrate and lipid levels, inflammatory marker, also prevented associated inflammation and heart damage.









Similar content being viewed by others
References
Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA. 2015;313(19):1973–4.
Bernadette Boden-Albala Community Level Disadvantage and the Likelihood of First Ischemic Stroke, Epidemiology Research International. Hindawi Publishing Corporation, 2012 Published online https://doi.org/10.1155/2012/481282
Low Wang C., Hess C., Hiatt W. and Goldfine A. Atherosclerotic cardiovasculr disease and heart failure in type 2 diabetes – mechanisms, management, and clinical considerations. Circulation. 2016; 133(24): 2459–2502. 10.1161.
Fall T, Ingelsson E. Genome-wide association studies of obesity and metabolic syndrome. Mol Cell Endocrinol. 2014;382(1):740–57. https://doi.org/10.1016/j.mce.2012.08.018.
Pap A, Cuaranta-Monroy I, Peloquin M, Nagy L. Is the mouse a good model of human PPARγ-related metabolic diseases? Int J Mol Sci. 2016;17(8):1236.
Kintscher U, Goebel M. INT-131, a PPAR-γ agonist for the treatment of type 2 diabetes. Curr Opin Investig Drugs. 2009;10(4):381–7.
Gobato AO, Vasques AC, Zambon MP, de Azevedo Barros A, Hessel G. Metabolic syndrome and insulin resistance in obese adolescents. Rev Paul Pediatr. 2014;32(1):55–62.
Krikun G, Trezza J, Shaw J, Rahman M, Guller S, Abrahams VM, et al. Lipopolysaccharide appears to activate human endometrial endothelial cells through TLR-4-dependent and TLR-4-independent mechanisms. Am J Reprod Immunol. 2012;68:233–7.
Ren Z, Zhao A, Wang Y, Meng L, Man-Yau SI. Association between dietary inflammatory index, C-reactive protein and metabolic syndrome: a cross-sectional study. Nutrients. 2018;10(7):831.
Lu P, Sodhi CP, Yamaguchi Y, Jia H, Prindle T Jr, Fulton WB, et al. Intestinal epithelial toll-like receptor 4 prevents metabolic syndrome by regulating interactions between microbes and intestinal epithelial cells in mice. Mucosal Immunol. 2018;11(3):727–40. https://doi.org/10.1038/mi.2017.114.
Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97.
Cotillard A, Poitou C, Torcivia A, Bouillot JL, Dietrich A, Kloting N, et al. Adipocyte size threshold matters: link with risk of type 2 diabetes and improved insulin resistance after gastric bypass. J Clin Endocrinol Metab. 2014;99:E1466–70. https://doi.org/10.1210/jc.2014-1074.
Olson AL. Regulation of GLUT4 and insulin-dependent glucose flux. ISRN Mol Biol. 2012;2012:856987. https://doi.org/10.5402/2012/856987.
Ferreira DF, Fiamoncini J, Prist IH, Ariga SK, de Souza HP, de Lima TM. Novel role of TLR4 in NAFLD development: modulation of metabolic enzymes expression. Biochim Biophys Acta. 2015;1851(10):1353–9. https://doi.org/10.1016/j.bbalip.2015.07.002.
Shi Y, Jia M, Xu L, Fang Z, et al. miR-96 and autophagy are involved in the beneficial effect of grape seed proanthocyanidins against high-fat-diet-induced dyslipidemia in mice. Phytother Res. 2019;8. https://doi.org/10.1002/ptr.6318.
Sanna RS, Muthangi S, Devi SA. Grape seed proanthocyanidin extract and insulin prevents cognitive decline in type 1 diabetic rat by impacting Bcl-2 and Bax in the prefrontal cortex. Metab Brain Dis. 2019;34(1):103–17. https://doi.org/10.1007/s11011-018-0320-5.Epub2018.
Pascual-Serrano A, Bladé C, Suárez M, Arola-Arnal A. Proanthocyanidins improve white adipose tissue expansion during diet-induced obesity development in rats. Int J Mol Sci. 2018:E2632. https://doi.org/10.3390/ijms19092632.
Samodien E., Pheiffer C., Erasmus M., Mabasa L, Louw J, Johnson R. Diet-induced DNA methylation within the hypothalamic arcuate nucleus and dysregulated leptin and insulin signaling in the pathophysiology of obesity Food Science and Nutrition. – 2019. 7(10). – 2019. – P.3131–3145. https://doi.org/10.1002/fsn3.1169
Salehi B, Mishra A, Nigam M et al. Resveratrol: a double-edged sword in health benefits. Biomedicines. 2018;6(3):91. Published 2018 Sep 9. https://doi.org/10.3390/biomedicines6030091
Galiniak S, Aebisher D, Bartusik-Aebisher D. Health benefits of resveratrol administration. Acta Biochim Pol. 2019;66. https://doi.org/10.18388/abp.2018_2749.
Rahimifarda M, Shermineh F, Moeini-Nodeh S, et al. Targeting the TLR4 signaling pathway by polyphenols: a novel therapeutic strategy for neuroinflammation. Ageing Res Rev. 2017;36:11–9.
Hajiaghaalipour F, Khalilpourfarshbafi M, Arya A. Modulation of glucose transporter protein by dietary flavonoids in type 2 diabetes mellitus. Int J Biol Sci. 2015;11(5):508–524. Published 2015 Mar 19. https://doi.org/10.7150/ijbs.11241
Asgary S, Karimi R, Momtaz S, Naseri R, Farzaei MH. Effect of resveratrol on metabolic syndrome components: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2019;20:173–86. https://doi.org/10.1007/s11154-019-09494-z.
Park JH, Kho MC, Kim HY, Ahn YM, Lee YJ, Kang DG, Lee HS. Blackcurrant suppresses metabolic syndrome induced by high-fructose diet in rats. Evid Based Complement Alternat Med. 2015; Published online 2015 Oct 4. https://doi.org/10.1155/2015/385976, 1, 11.
Chou CL, Lai YH, Lin TY, Lee TJ, Fang TC. Renin inhibition improves metabolic syndrome, and reduces angiotensin II levels and oxidative stress in visceral fat tissues in fructose-fed rats. PLoS One. 2017;12(7):e0180712. https://doi.org/10.1371/journal.pone.0180712.
FDA Briefing Document, Pharmacy Compounding Advisory Committee (PCAC) Meeting, 2017.
Alberti KG, Zimmet P, Shaw J. IDF epidemiology task force consensus group. The metabolic syndrome a new worldwide definition. Lancet. 2005;366:1059–62.
Alberti KG, Zimmet P, Shaw J. Metabolic syndrome-a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006 May;23(5):469–80.
Purcell SH, Aerni-Flessner LB, Willcockson AR, Diggs-Andrews KA, Fisher SJ, Moley KH. Improved insulin sensitivity by GLUT12 overexpression in mice. Diabetes. 2011 May;60(5):1478–82.
Zhou QL, Park JG, Jiang ZY, Holik JJ, Mitra P, Semiz S, et al. Analysis of insulin signalling by RNAi-based gene silencing. Biochem Soc Trans. 2004 Nov;32(Pt 5):817–21.
Kubyshkin A, Ogai Y, Fomochkina I, Chernousova I, et al. Polyphenols of red grape wines and alcohol-free food concentrates in rehabilitation technologies. Polyphenols. 2018:99–120. https://doi.org/10.5772/intechopen.76655.
Han L, Shen WJ, Bittner S, Kraemer FB, Azhar S. PPARs: regulators of metabolism and as therapeutic targets in cardiovascular disease. Part II: PPAR-β/δ and PPAR-γ Future Cardiol. 2017;3:279–96. https://doi.org/10.2217/fca-2017-0019.Epub2017.
Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, et al. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19(5):557–66. https://doi.org/10.1038/nm.3159.Epub 2013.
Suhett LG, Hermsdorff HHM, Rocha NP, Silva MA, Filgueiras MDS, Milagres LC, et al. Increased C-reactive protein in Brazilian children: association with Cardiometabolic risk and metabolic syndrome components (PASE study). Cardiol Res Pract. 2019;3904568:1–10. https://doi.org/10.1155/2019/3904568.
Reddya P, Lent-Schocheta D, Ramakrishnana N, McLaughlina M, Jialalab I. Metabolic syndrome is an inflammatory disorder: a conspiracy between adipose tissue and phagocytes. Clin Chim Acta. 2019;496:35–44. https://doi.org/10.1016/j.cca.2019.06.019.
Kim MH, et al. The association between subclinical inflammation and abnormal glucose and lipid metabolisms in normal-weight Korean individuals. Nutr Metab Cardiovasc Dis. 28(11):1106–13.
Anderson PD, Mehta NN, Wolfe ML, et al. Innate immunity modulates adipokines in humans. J Clin Endocrinol Metab. 2007;92(6):2272–9.
Petrenko VI, Kubyshkin AV, Fomochkina II, Kucherenko AS, Aliev LL, Sorokina LE, et al. Study of the pathogenetic mechanisms of the pleiotropic action of angiotensin II type 1 receptor blockers in metabolic syndrome. NAMJ. 2019;13(2):18–23.
Li Y, Wende AR, Nunthakungwan O, Huang Y, Hu E, Jin H, et al. Cytosolic, but not mitochondrial, oxidative stress is a likely contributor to cardiac hypertrophy resulting form cardiac specific GLUT4 deletion in mice. FEBS J. 2012;279:599–611.
Funding
This work was partially supported by the V.I. Vernadsky Crimean Federal University Development Program for 2015–2024.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kubyshkin, A., Shevandova, A., Petrenko, V. et al. Anti-inflammatory and antidiabetic effects of grape-derived stilbene concentrate in the experimental metabolic syndrome. J Diabetes Metab Disord 19, 1205–1214 (2020). https://doi.org/10.1007/s40200-020-00626-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-020-00626-w