Abstract
Purpose
Diabetes mellitus is associated with perturbations in brain biochemical parameters associated with dementia. This study aimed at comparing the effect of metformin and metformin/donepezil combination on oxidative stress, endoplasmic reticulum stress and inflammation in the brain of diabetic Wistar rats.
Methods
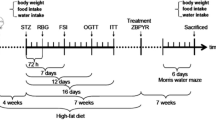
Diabetes was induced by single intraperitoneal injection of 40 mg/kg streptozotocin after administration of 10% fructose for 14 days. Animals were randomly assigned to four groups of five animals each. Group 1 was the normal control and received only distilled water. Groups 2 and 3 were diabetic rats treated with metformin/donepezil combination and metformin only respectively, while group 4 was diabetic control. Treatment lasted for 21 days after confirmation of diabetes. Activities of acetylcholinesterase (AchE), butyrylcholinesterase (BchE), superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase were evaluated in the brain of diabetic rats. Enzyme-linked immunosorbent assay was used to estimate brain levels of tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6) malondialdehyde and glucose transporter-4 (GLUT4), while expression of endoplasmic reticulum stress markers – glucose regulated protein-78 (GRP78), activating transcription factor-4 (ATF4) and C/EBP homologous protein (CHOP) was determined using real-time PCR in the hippocampus of diabetic rats.
Results
Treatment with metformin/donepezil combination significantly reduced the activities of AchE, BchE as well as levels of malondialdehyde, TNF-α and IL-6, while the activities of SOD, GPx and catalase were significantly increased in the brain. Moreover, expression of ER stress markers was attenuated in the hippocampus.
Conclusion
Metformin/donepezil combination appeared more efficacious than metformin only and could be considered for managing diabetes-associated dementia.













Similar content being viewed by others
Data availability
Available on request.
References
Zhang Z, Fang P, Shia M, Zhu Y, Bo P. Elevated galanin may predict the risk of type 2 diabetes mellitus for development of Alzheimer’s disease. Mech Ageing Dev. 2015;150:20–6.
Ascher-Svanum H, Chen YF, Hake A, Kahle-Wrobleski K, Schuster D, Kendall D, et al. Cognitive and functional decline in patients with mild Alzheimer dementia with or without comorbid diabetes. Clin Ther. 2015;37(6):1195–205.
Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen R, O’Brien PC, Palumbo PJ. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol. 1997;14:301–8.
Rosenberg PB. Clinical aspects of inflammation in Alzheimer’s disease. Int Rev Psychiatry. 2005;17:503–14.
Takeda S, Sato N, Uchio-Yamada K (2010) Diabetes accelerated memory dysfunction via cerebrovascular inflammation and Aβ deposition in Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A 107:7036–41
Das UN. Acetylcholinesterase and butyrylcholinesterase as markers of low-grade systemic inflammation. Ann Hepatol. 2012;11(3):409–11.
Mushtaq G, Greig NH, Khan JA, Kamal MA. Status of Acetylcholinesterase and Butyrylcholinesterase in Alzheimer’s Disease and Type 2 Diabetes Mellitus. CNS Neurol Disord Drug Targets. 2014;13(8):1432–9.
Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3:169–78.
Takeda S, Sato N, Rakugi H, Morishita R. Molecular mechanisms linking diabetes mellitus and Alzheimer disease: Beta-amyloid peptide, insulin signaling, and neuronal function. Mol Biosyst. 2011;7:1822–7.
Verdile G, Fuller SJ, Martins RN. The role of type 2 diabetes in neurodegeneration. Neurobiol Dis. 2015;84:22–38.
Talbot K, Wang HY, Kazi H, Han L, Bakshi KP, Stucky A. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122(4):1316–38.
Benedict C, Grillo CA. Insulin resistance as a therapeutic target in the treatment of Alzheimer’s disease: a state-of-the-art review. Front Neurosci. 2018;12:215.
Wallberg-Henriksson H, Zierath JR. GLUT4: a key player regulating glucose homeostasis? Insights from transgenic and knockout mice (review). Mol Membr Biol. 2001;18:205–11.
Vannucci SJ, Koehler-Stec EM, Li K, Reynolds TH, Clark R, Simpson IA. Glut4 glucose transporter expression in rodent brain: Effect of diabetes. Brain Res. 1998;797:1–11.
Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13(2):89–102.
Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–32.
Kind KR, Ball KK, Cruz NF, Dienel GA. The unfolded protein response to endoplasmic reticulum stress in cultured astrocytes and rat brain during experimental diabetes. Neurochem Int. 2013;62(5):784–95.
Schroder M. Endoplasmic reticulum stress responses. Cell Mol Life Sci. 2008;65:862–94.
Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–77.
Scheper W, Hoozemans JJ. Endoplasmic reticulum protein quality control in neurodegenerative disease: the good, the bad and the therapy. Curr Med Chem. 2009;16:615–26.
Salminen A, Kauppinen A, Suuronen T, kaarniranta K, Ojala J. ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. J Neuroinflammation. 2009;6:41.
Duran-Aniotz C, Cornejo VH, Espinoza S, Ardiles AO, Medinas DB, Salazar C, Foley A, Gajardo I, Thielen P, Iwawaki T, Scheper W, Soto C, Palacios AG, Hoozemans JJM, Hetz C. IRE1 signaling exacerbates Alzheimer’s disease pathogenesis. Acta Neuropathol. 2017:1e18.
Hamos JE, Oblas B, Pulaski-Salo D, Welch WJ, Bole DG, Drachman DA. Expression of heat shock proteins in Alzheimer’s disease. Neurology. 1991;41:345e350.
Hoozemans JJM, van Haastert ES, Nijholt DAT, Rozemuller AJM, Scheper W. Activation of the unfolded protein response is an early event in Alzheimer’s and Parkinson’s disease. Neurodegener Dis. 2012;10:212e215.
Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, Urano F. Regulation of insulin biosynthesis in pancreatic b cells by an endoplasmic reticulum- resident protein kinase IRE1. Cell Metab. 2006;4:245–54.
Kuca K, Soukup O, Maresova P, Korabecny J, Nepovimova E, Kilmova B. Current approaches against Alzheimer’s disease in clinical trials. J Braz Chem Soc. 2016;27:641–9.
Kumar K, Kumar A, Keegan RM, Deshmukh R. Recent advances in the neurobiology and neuropharmacology of Alzheimer’s disease. Biomed Pharmacother. 2018;98:297–307.
Cacabelos R. Donepezil in Alzheimer’s disease: From conventional trials to pharmacogenetics. Neuropsychiatr Dis Treat. 2007;3:303–33.
Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci. 2012;1;22:253–70.
Rotermund C, Machetanz G, Fitzgerald JC. The Therapeutic Potential of Metformin in Neurodegenerative Diseases. Front Endocrinol. 2018;9:400.
Yokoyama H, Ogawa M, Honjo J, Okizaki S, Yamada D, Shudo R, et al. Risk factors associated with abnormal cognition in Japanese outpatients with diabetes, hypertension or dyslipidemia. Diabetol Int. 2015;6:268–74.
Orkaby AR, Cho K, Cormack J, Gagnon DR, Driver JA. Metformin vs sulfonylurea use and risk of dementia in US veterans aged >/=65 years with diabetes. Neurology. 2017;89:1877–85.
Dong L, Zhou Q, Zhang Z, Zhu Y, Duan T, Feng Y. Metformin sensitizes endometrial cancer cells to chemotherapy by repressing glyoxalase I expression. J Obstet Gynaecol Res. 2012;38:1077–85.
Wilson RD, Islam MS. Fructose-fed streptozotocin-injected rat: an alternative model for type 2 diabetes. Pharmacol Rep. 2012;64:129–39.
Tsakiris S, Schulpis KH, Marinou K, Behrakis P. Protective effect of L-cysteine and glutathione on the modulated suckling rat brain Na+K+–ATPase and Mg2+-ATPase activities induced by the in vitro galactosaemia. Pharmacol Res. 2004;49:475–9.
Guide for the care and use of laboratory animals. 8th ed. The National Academic Press, Washington, DC 20001; 2011.
Varshney R, Kale RK. Effect of calmodulin antagonists on radiation induced lipid peroxidation in microsomes. Int J Radiat Biol. 1990;58:733–43.
Misra HP, Fridovich I. The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5.
Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47(2):389–94.
Rotruck JT, Pope AL, Ganther HE, Swason AB. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588e90.
Ali MA, Rizvi S, Syed BA. Trends in the market for antihypertensive drugs. Nat Rev Drug Discov. 2017;16:309–10.
Cheng F, Kovacs IA, Barabasi A. Network-based prediction of drug combinations. Nat Commun. 2019:1–11. https://doi.org/10.1038/s41467-019-09186-x.
Vincent C, Hall PA. Executive function in adults with type 2 diabetes: a meta-analytic review. Psychosom Med. 2015;77(6):631–42.
Cooper C, Sommerlad A, Lyketsos CG, Livingston G. Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta-analysis. Am J Psychiatry. 2015;172:323–34.
Gomez AM, Umpierrez GE. Continuous glucose monitoring in insulin-treated patients in non-ICU settings. J Diabetes Sci Technol. 2014;8:930–6.
Diehl T, Mullins R, Kapogiannis D. Insulin resistance in Alzheimer’s disease. Transl Res. 2017;183:26–40.
Kamal MA, Greig NH, Reale M. Anti-inflammatory properties of acetylcholinesterase inhibitors administered in Alzheimer’s disease. Anti Inflamm Anti-Allergy Agents Med Chem. 2009;8(1):85–100.
Nordberg A, Ballard C, Bullock R, Darreh-Shori T, Somogyi M. A review of butyrylcholinesterase as a therapeutic target in the treatment of Alzheimer’s disease. Prim Care Companion CNS Disord. 2013;15(2):PCC.12r01412.
Saliua JA, Oboh G, Omojokun OS, Rochad J, Schetinger MR, Guterries J, et al. Effect of dietary supplementation of Padauk (Pterocarpus soyauxii) leaf on high fat diet/streptozotocin induced diabetes in rats’ brain and platelets. Biomed Pharmacother. 2016;84:1194–201.
Markowicz-Piasecka M, Huttunen KM, Sikora J. Metformin and its sulphonamide derivative simultaneously potentiateanti-cholinesterase activity of donepezil and inhibit beta-amyloid aggregation. J Enzyme Inhib Med Chem. 2018;33(1):1309–22.
Butterfield DA, Poon HF, St Clair D, Keller JN, Pierce WM, Klein JB. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer’s disease. Neurobiol Dis. 2006;22:223–32.
Maciejczyk M, Zebrowska E, Zalewska A, Chabowski A (2018) Redox, balance, antioxidant defense, and oxidative damage in the hypothalamus and cerebral cortex of rats with high fat diet- induced insulin resistance. Oxid Med Cell Longev 2018:1–11
Cobley JN, Fiorello ML, Bailey DM. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018;15:490–503.
Marksbery WR. The role of oxidative stress in Alzheimer’s disease. Arch Neurol. 1999;56:1449–52.
Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–66.
Flekac M, Skrha J, Hilgertova J, Lacinova Z, Jarolimkova M. Gene polymorphisms of superoxide dismutases and catalase in diabetes mellitus. BMC Med Genet. 2008;9:30–5.
Clark GJ, Pandya K, Lau-Cam CA. The effect of metformin and taurine, alone and in combination, on the oxidative stress caused by diabetes in the rat brain. Adv Exp Med Biol. 2017;975(1):353–69.
Chatterjee S, Mudher A. Alzheimer’s disease and type 2 diabetes: A critical assessment of the shared pathological traits. Front Neurosci. 2018;12:383.
Decourt B, Lahiri DK, Sabbagh MN. Targeting tumor necrosis factor alpha for alzheimer’s disease. Curr Alzheimer Res. 2017;14(4):412–25.
Obafemi TO, Olaleye MT, Akinmoladun AC. Antidiabetic property of miracle fruit plant (Synsepalum dulcificum Shumach. & Thonn. Daniell) leaf extracts in fructose-fed streptozotocin-injected rats via anti- inflammatory activity and inhibition of carbohydrate metabolizing enzymes. J. Ethnopharmacol. 2019;244:112124. https://doi.org/10.1016/j.jep.2019.112124.
Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16(6):358–72.
Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor κB activation via AMPactivated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47:1183–8.
Brambilla R, Hurtado A, Persaud T, Esham K, Pearse DD, Oudega M, et al. Transgenic inhibition of astroglial NF-κB leads to increased axonal sparing and sprouting following spinal cord injury. J Neurochem. 2009;110:765–78.
Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic infl ammatory response to endotoxin. Nature. 2000;405:458–62.
Pearson-Leary J, McNay EC. Novel roles for the insulin-regulated glucose transporter- 4 in hippocampally dependent memory. J Neurosci. 2016;36:11851–64. 42.
Ashrafi G, Wu Z, Farrell RJ, Ryan TA. GLUT4 mobilization supports energetic demands of active synapses. Neuron. 2017;93:606–15.
Ngarmukos C, Baur EL, Kumagai AK. Co-localization of glut1 and glut4 in the blood- brain barrier of the rat ventromedial hypothalamus. Brain Res. 2001;900:1–8.
Mehta V, Parashar A, Sharma A, Singh TR, Udayabanu M. Quercetin ameliorates chronic unpredicted stress-mediated memory dysfunction in male Swiss albino mice by attenuating insulin resistance and elevating hippocampal GLUT4 levels independent of insulin receptor expression. Horm Behav. 2017;89:13–22.
Cheng J, Huang C, Liu I, Tzeng T, Chang C. Novel mechanism for plasma glucose– lowering action of metformin in streptozotocin-induced diabetic rats. Diabetes. 2006;55:819–25.
Lu J, Wu DM, Zheng YL, Hu B, Cheng W, Zhang ZF, Shan Q. Ursolic acid improves high fat diet-induced cognitive impairments by blocking endoplasmic reticulum stress and IκB kinase β/nuclear factor-κB-mediated inflammatory pathways in mice. Brain Behav Immun. 2011;25:1658–67.
Ye T, Meng X, Wang R, Zhang C, He S, Sun G, Sun X. Gastrodin alleviates cognitive dysfunction and depressive-like behaviors by inhibiting ER stress and NLRP3 inflammasome activation in db/db mice. Int J Mol Sci. 2018;19(12):3977.
Imaizumi K, Miyoshi K, Katayama T, Yoneda T, Taniguchi M, Kudo T, Tohyama M. The unfolded protein response and Alzheimer’s disease. Biochim Biophys Acta. 2001;1536:85–96.
Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA. 2004;101:11269–74.
Wang XZ, Kuroda M, Sok J, Batchvarova N, Kimmel R, Chung P, Zinszner H, et al. Identification of novel stress-induced genes downstream of CHOP. EMBO J. 1998;17:3619–33.
Zhang X, Xu L, He D, Ling S. Endoplasmic reticulum stress-mediated hippocampal neuron apoptosis involved in diabetic cognitive impairment. Biomed Res Int. 2013:1–9.
Hoozemans JJM, Veerhuis R, Van Haastert ES, Rozemuller JM, Baas F, Eikelenboom P, Scheper W. The unfolded protein response is activated in Alzheimer’s disease. Acta Neuropathol. 2005;110(2):165–72.
Ohno M. Roles of eIF2alpha kinases in the pathogenesis of Alzheimer’s disease. Front Mol Neurosci. 2014;7:22.
Huang H, Jing G, Wang JJ, Sheibani N, Zhang SX. ATF4 is a novel regulator of MCP-1 in microvascular endothelial cells. J Inflamm (Lond). 2015;12:31.
Chi H, Chang H, Sang T. Neuronal cell death mechanisms in major neurodegenerative diseases. Int J Mol Sci. 2018;19:3082.
Shruthi K, Reddy SS, Chitra PS, Reddy GB. Ubiquitin-proteasome system and ER stress in the brain of diabetic rats. J Cell Biochem. 2018:1–12. https://doi.org/10.1002/jcb.27884.
Lagali PS, Corcoran CP, Picketts DJ. Hippocampus development and function: role of epigenetic factors and implications for cognitive disease. Clin Genet. 2010;78:321–33.
Funding
Authors did not receive any funding from institutions or individuals for this research.
Author information
Authors and Affiliations
Contributions
Obafemi T. O. and Olasehinde O. R designed the study; Olaoye O. A, Jaiyesimi K. F and Adewumi D.F. did most of the bench work; Afolabi B. A prepared the first draft of the manuscript; Adewale O. B proof-read and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
An ethical approval with number ABUAD-SCI19/03/102 was obtained from Animal Care Committee of the Afe Babalola University Research Directorate, Ado-Ekiti, Nigeria.
Consent to participate
Not applicable.
Consent for publication
All authors approved the submission of the manuscript for publication
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Obafemi, T.O., Olasehinde, O.R., Olaoye, O.A. et al. Metformin/Donepezil combination modulates brain antioxidant status and hippocampal endoplasmic reticulum stress in type 2 diabetic rats. J Diabetes Metab Disord 19, 499–510 (2020). https://doi.org/10.1007/s40200-020-00541-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-020-00541-0