Abstract
Background
Renal cell carcinoma (RCC) is a common malignancy. Local anesthetics were displayed powerful effects against various cancers. This study aims to probe the functions and molecular mechanism of ropivacaine in RCC.
Methods
Different concentrations of ropivacaine were performed to administrate RCC cells including 786-O and Caki-1 cells. Cell viability and cell apoptosis were examined using CCK-8 and flow cytometry, respectively. Cell migration and invasion were determined by transwell assay. RMRP and CCDC65 expression was firstly predicted using TCGA dataset and further validated in RCC cells using qRT-PCR and western blot. The interactions among RMRP, EZH2 and CCDC65 were verified by RNA immunoprecipitation (RIP) and chromatin immunoprecipitation (ChIP) assays.
Results
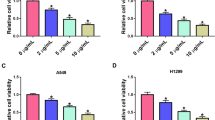
Ropivacaine effectively suppressed RCC cell viability, migration and invasion and enhanced cell apoptosis rate. Aberrantly elevated RMRP expression in RCC tissues was predicted by TCGA database. Interestingly, overexpressed RMRP observed in RCC cells could be also blocked upon the administration of ropivacaine. Likewise, RMRP knockdown further strengthened ropivacaine-mediated tumor suppressive effects on RCC cells. In terms of mechanism, RMRP directly interacted with EZH2, thereby modulating the histone methylation of CCDC65 to silence its expression. Moreover, ropivacaine inhibited tumor growth in mice bearing RCC tumor through regulating RMRP/EZH2/CCDC65 axis.
Conclusion
In sum up, our work revealed that ropivacaine suppressed capacities of RCC cell viability, migration and invasion through modulating the RMRP/EZH2/CCDC65 axis, which laid the experimental foundation of ropivacaine for clinical application in the future.
Graphical Abstract








Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Klatte T, Rossi SH, Stewart GD. Prognostic factors and prognostic models for renal cell carcinoma: a literature review. World J Urol. 2018;36(12):1943–52.
Müller SD, Ziegler JSH, Piegeler T. Local anesthetics and recurrence after cancer surgery-what’s new? a narrative review. J Clin Med. 2021;10(4):719.
Bharati SJ, et al. Anesthetics impact on cancer recurrence: what do we know? J Cancer Res Ther. 2016;12(2):464–8.
Ni J, et al. Amide-linked local anesthetics preferentially target leukemia stem cell through inhibition of wnt/β-catenin. Biochem Biophys Res Commun. 2018;503(2):956–62.
Wang W, et al. Ropivacaine promotes apoptosis of hepatocellular carcinoma cells through damaging mitochondria and activating caspase-3 activity. Biol Res. 2019;52(1):36.
Dan J, et al. Inhibition of gastric cancer by local anesthetic bupivacaine through multiple mechanisms independent of sodium channel blockade. Biomed Pharmacother. 2018;103:823–8.
Piegeler T, et al. Clinically relevant concentrations of lidocaine and ropivacaine inhibit TNFα-induced invasion of lung adenocarcinoma cells in vitro by blocking the activation of Akt and focal adhesion kinase. Br J Anaesth. 2015;115(5):784–91.
Zheng Q, Peng X, Yu H. Local anesthetic drug inhibits growth and survival in chronic myeloid leukemia through suppressing PI3K/Akt/mTOR. Am J Med Sci. 2018;355(3):266–73.
Li C, et al. Procaine inhibits the proliferation and migration of colon cancer cells through inactivation of the ERK/MAPK/FAK pathways by regulation of RhoA. Oncol Res. 2018;26(2):209–17.
Simpson D, et al. Ropivacaine: a review of its use in regional anaesthesia and acute pain management. Drugs. 2005;65(18):2675–717.
Wang X, Li T. Ropivacaine inhibits the proliferation and migration of colorectal cancer cells through ITGB1. Bioengineered. 2021;12(1):44–53.
Zhang N, et al. Ropivacaine Inhibits the Growth, Migration and Invasion of Gastric Cancer Through Attenuation of WEE1 and PI3K/AKT Signaling via miR-520a-3p. Onco Targets Ther. 2020;13:5309–21.
Bridges MC, Daulagala AC, Kourtidis A. LNCcation: lncRNA localization and function. J Cell Biol. 2021;220(2):e202009045.
Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77(15):3965–81.
Liu Y, et al. Potential role of lncRNA H19 as a cancer biomarker in human cancers detection and diagnosis: A Pooled Analysis Based on 1585 Subjects. Biomed Res Int. 2019;2019:9056458.
Liu S, et al. Identification of the 3-lncRNA signature as a prognostic biomarker for colorectal cancer. Int J Mol Sci. 2020;21(24):9359.
Hussen BM, et al. Long non-coding RNA RMRP in the pathogenesis of human disorders. Front Cell Dev Biol. 2021;9:676588.
Cao HL, et al. lncRNA-RMRP promotes proliferation, migration and invasion of bladder cancer via miR-206. Eur Rev Med Pharmacol Sci. 2019;23(3):1012–21.
Hongfeng Z, et al. lncRNA RMRP knockdown suppress hepatocellular carcinoma biological activities via regulation miRNA-206/TACR1. J Cell Biochem. 2020;121(2):1690–702.
Bachmann IM, et al. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol. 2006;24(2):268–73.
Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469(7330):343–9.
Hui B, et al. RREB1-induced upregulation of the lncRNA AGAP2-AS1 regulates the proliferation and migration of pancreatic cancer partly through suppressing ANKRD1 and ANGPTL4. Cell Death Dis. 2019;10(3):207.
Priyanka PP, Yenugu S. Coiled-coil domain-containing (CCDC) proteins: functional roles in general and male reproductive physiology. Reprod Sci. 2021;28(10):2725–34.
Liu Z, et al. Regulatory network and targeted interventions for CCDC family in tumor pathogenesis. Cancer Lett. 2023;565: 216225.
Deng T, et al. CCDC65 as a new potential tumor suppressor induced by metformin inhibits activation of AKT1 via ubiquitination of ENO1 in gastric cancer. Theranostics. 2021;11(16):8112–28.
Zhang Z, et al. CCDC65, a gene knockout that leads to early death of mice, acts as a potentially novel tumor suppressor in lung adenocarcinoma. Int J Biol Sci. 2022;18(10):4171–86.
Le Gac G, et al. Local anesthetics inhibit the growth of human hepatocellular carcinoma cells. Anesth Analg. 2017;125(5):1600–9.
Zhang Y, et al. Mechanisms of cancer inhibition by local anesthetics. Front Pharmacol. 2021;12: 770694.
Xu P, et al. Local anesthetic ropivacaine exhibits therapeutic effects in cancers. Front Oncol. 2022;12: 836882.
Charles Richard JL, Eichhorn PJA. Platforms for investigating LncRNA functions. SLAS Technol. 2018;23(6):493–506.
Liu R, et al. Ropivacaine inhibits proliferation, migration, and invasion while inducing apoptosis of glioma cells by regulating the SNHG16/miR-424-5p axis. Open Life Sci. 2020;15(1):988–99.
Lu Y, et al. Ropivacaine retards the viability, migration, and invasion of choriocarcinoma cells by regulating the long noncoding RNA OGFRP1/MicroRNA-4731-5p/HIF3A axis. Mol Biotechnol. 2022;64(5):499–509.
Xie Z, et al. lncRNA RMRP predicts poor prognosis and mediates tumor progression of esophageal squamous cell carcinoma by regulating miR-613/ neuropilin 2 (NRP2) axis. Bioengineered. 2021;12(1):6913–22.
Yang M, Ke H, Zhou W. LncRNA RMRP promotes cell proliferation and invasion through miR-613/NFAT5 axis in non-small cell lung cancer. Onco Targets Ther. 2020;13:8941–50.
da Rocha ST, et al. Jarid2 is implicated in the initial xist-induced targeting of PRC2 to the inactive X chromosome. Mol Cell. 2014;53(2):301–16.
Laugesen A, Højfeldt JW, Helin K. Molecular mechanisms directing PRC2 recruitment and H3K27 methylation. Mol Cell. 2019;74(1):8–18.
Wang X, et al. A lncRNA coordinates with Ezh2 to inhibit HIF-1α transcription and suppress cancer cell adaption to hypoxia. Oncogene. 2020;39(9):1860–74.
Fang P, et al. LncRNA LINC00525 suppresses p21 expression via mRNA decay and triplex-mediated changes in chromatin structure in lung adenocarcinoma. Cancer Commun (Lond). 2021;41(7):596–614.
Guo L, et al. CCDC137 Is a prognostic biomarker and correlates with immunosuppressive tumor microenvironment based on pan-cancer analysis. Front Mol Biosci. 2021;8:674863.
Funding
This work was supported by Natural Science Foundation of Jiangxi Province(20192BAB205041).
Author information
Authors and Affiliations
Contributions
Yingfen Xiong: acquisition of data; analysis and interpretation of data; drafting the manuscript.
Xiaolan Zheng: acquisition of data; drafting the manuscript.
Huangying Deng: conception and design of study; revising the manuscript critically for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interests
These authors declared no competing interests in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. Ropivacaine evidently inhibited the capacities of RCC cell viability, migration and invasion.
2. Ropivacaine exhibited anti-tumor biological effects on RCC cells via downregulating RMRP expression.
3. RMRP epigenetically repressed CCDC65 expression via recruiting EZH2.
4. Ropivacaine inhibited tumor growth of mice bearing RCC tumor through regulating the RMRP/EZH2/CCDC65 axis.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiong, Y., Zheng, X. & Deng, H. Ropivacaine suppresses the progression of renal cell carcinoma through regulating the lncRNA RMRP/EZH2/CCDC65 axis. DARU J Pharm Sci 32, 121–132 (2024). https://doi.org/10.1007/s40199-023-00492-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-023-00492-w