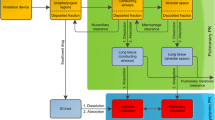
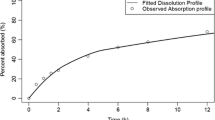
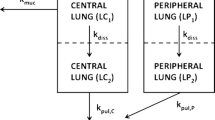
Abstract
Objectives
There are a multitude of different modelling techniques that have been used for inhaled drugs. The main objective of this review was to conduct an exhaustive survey of published mathematical models in the area of asthma and chronic obstructive pulmonary disease (COPD) for inhalation drugs. Additionally, this review will attempt to assess the applicability of these models to assess bioequivalence (BE) of orally inhaled products (OIPs).
Evidence acquisition
PubMed, Science Direct, Web of Science, and Scopus databases were searched from 1996 to 2020, to find studies that described mathematical models used for inhaled drugs in asthma/COPD.
Results
50 articles were finally included in this systematic review. This research identified 22 articles on in silico aerosol deposition models, 20 articles related to population pharmacokinetics and 8 articles on physiologically based pharmacokinetic modelling (PBPK) modelling for inhaled drugs in asthma/COPD. Among all the aerosol deposition models, computational fluid dynamics (CFD) simulations are more likely to predict regional aerosol deposition pattern in human respiratory tracts. Across the population PK articles, body weight, gender, age and smoking status were the most common covariates that were found to be significant. Further, limited published PBPK models reported approximately 29 parameters relevant for absorption and distribution of inhaled drugs. The strengths and weaknesses of each modelling technique has also been reviewed.
Conclusion
Overall, while there are different modelling techniques that have been used for inhaled drugs in asthma and COPD, there is very limited application of these models for assessment of bioequivalence of OIPs. This review also provides a ready reference of various parameters that have been considered in various models which will aid in evaluation if one model or hybrid in silico models need to be considered when assessing bioequivalence of OIPs.
Graphical abstract



Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ACI:
-
Andersen Cascade Impactor
- BE:
-
Bioequivalence
- CFD:
-
Computational fluid dyanamics
- COPD:
-
Chronic Obstructive Pulmonary Disease
- IVIVC/E:
-
In vitro in vivo Correlation/extrapolation
- IP:
-
Induction port
- NGI:
-
New generation impactor
- OFV:
-
Objective function value
- PBPK:
-
Physiologically based pharmacokinetics
- PD:
-
Pharmacodynamics
- PK:
-
Pharmacokinetics
- VPC:
-
Visual predictive checks
References
Herriges M, Morrisey EE. Lung development: orchestrating the generation and regeneration of a complex organ. Dev (Camb). 2014;141(3):502–13.
Itoh H, Nishino M, Hatabu H. Architecture of the lung: morphology and function. J Thorac Imaging. 2004;19(4):221–7.
Longest PW. CFD and hybrid deposition modeling when and why the approach is useful. Respir Drug Deliv. 2018;1:123–36.
Delvadia R, Hindle M, Worth Longest P, Byron PR. In vitro tests for aerosol deposition II: IVIVCs for different dry powder inhalers in normal adults. J Aerosol Med Pulm Drug Deliv. 2013;26(3):138–44.
Wei X, Hindle M, Kaviratna A, Huynh BK, Delvadia RR, Sandell D, et al. In vitro tests for aerosol deposition. VI: Realistic testing with different mouth-throat models and in vitro - In vivo correlations for a dry powder inhaler, metered dose inhaler, and soft mist inhaler. J Aerosol Med Pulm Drug Deliv. 2018;31:1–14.
Longest PWHM. Small airway absorption and microdosimetry of inhaled corticosteriod particles after deposition. Pharm Res. 2017;34(10):2049–65.
Renishkumar R, Delvadia P. Worth longest and PRB. In vitro tests for aerosol deposition. I: scaling a physical model of the upper airways to predict drug deposition variation in normal humans. J Aerosol Med Pulm Drug Deliv. 2012;25(1):32–40.
Burnell PKP, Asking L, Borgström L, Nichols SC, Olsson B, Prime D, Shrubb I. Studies of the human oropharyngeal airspaces using magnetic resonance imaging IV–the oropharyngeal retention effect for four inhalation delivery systems. J Aerosol Med. 2007;20(3):269–81.
Stapleton KW, Guentsch E, Hoskinson MKFW. On the suitability of k-epsilon turbulence modeling for aerosol deposition in the mouth and throat. J Aerosol Sci. 2000;31(6):739–49.
Ruzycki CA, Martin AR, Finlay WH. An exploration of factors affecting in vitro deposition of pharmaceutical aerosols in the Alberta idealized throat. J Aerosol Med Pulm Drug Deliv. 2019;32(6):405–17.
Dissanayake S. Therapeutic equivalence for orally inhaled products in Europe: an update and counterpoint to the 505(j) approval process. Respir Drug Deliv. 2018;505:237–54.
Hocchaus G, Horhota S, Hendeles L, Suarez SRJ. Pharmacokinetics of orally inhaled drug products. AAPS J. 2015;17:769–75.
Jones HM, Parrott N, Jorga K, Lavé T. A novel strategy for physiologically based predictions of human pharmacokinetics. Clin Pharmacokinet. 2006;45(5):511–42.
Luttringer O, Theil F-P, Poulin P, Schmitt-Hoffmann AH, Guentert TW, Lavé T. Physiologically based pharmacokinetic (PBPK) modeling of disposition of epiroprim in humans. J Pharm Sci Res. 2003;92(10):1990–2007.
Longest PW, Holbrook LT. In silico models of aerosol delivery to the respiratory tract - development and applications. Adv Drug Deliv Rev. 2012;64(4):296–311.
Stahlhofen W, Rudolf GJA. Intercomparison of experimental regional aerosol deposition data. J Aerosol Med. 1989;2:285–308.
Finlay WHMA. Recent advances in predictive understanding of respiratory tract deposition. J Aerosol Med Pulm Drug Deliv. 2008;21:189–205.
Choi JKC. Mathematical analysis of particle deposition in human lungs: An improved single path transport model. Inhal Toxicol. 2007;19:925–39.
Asgharian B, Hofmann W, Bergmann R. Particle deposition in a multiple-path model of the human lung. Aerosol science and technology. Inhal Toxicol. 2001;34:332–9.
Katz I, Pichelin M, Caillibotte G, Montesantos S, Majoral C, Martonen T, Fleming J, Bennett MCJ. Controlled, parametric, individualized, 2D, and 3D imaging measurements of aerosol deposition in the respiratory tract of healthy human subjects: preliminary comparisons with simulations. Aerosol Sci Technol. 2013;47:714–23.
Conway J, Fleming J, Majoral C, Katz I, Perchet D, Peebles C, Tossici-Bolt L, Collier L, Caillibotte G, Pichelin M, Sauret-Jackson V, Martonen T, Apiou-Sbirlea G, Muellinger B, Kroneberg P, Gleske J, Scheuch G, Texereau J, Martin A, Montesantos SBM. Controlled, parametric, individualized, 2-D and 3-D imaging measurements of aerosol deposition in the respiratory tract of healthy human subjects for model validation. J Aerosol Sci. 2012;52:1–17.
Fleming JS, Epps BP, Conway JHMT. Comparison of SPECT aerosol deposition data with a human respiratory tract model. J Aerosol Med. 2006;19:268–78.
Koblinger L, Hofmann W. Monte Carlo modeling of aerosol deposition in human lungs. Part I: Simulation of particle transport in a stochastic lung structure. J Aerosol Sci. 1990;21:661–74.
Kim CS. Deposition of aerosol particles in human lungs: in vivo measurement and modeling. Biomarkers. 2009;14:54–8.
Martonen TB. Analytical model of hygroscopic particle behavior in human airways. Bull Math Biol. 1982;44(3):425–42.
Xi J, Longest PWMT. Effects of the laryngeal jet on nano- and microparticle transport and deposition in an approximate model of the upper tracheobronchial airways. J Appl Physiol. 2008;104(6):1761–77.
Longest PW, Vinchurkar SMT. Transport and deposition of respiratory aerosols in models of childhood asthma. J Aerosol Sci. 2006;37:1234–57.
Sznitman J, Heimshch T, Wildhaber JH, et al. Respiratory flow phenomena and gravitational deposition in a three-dimensional space-filling model of the pulmonary acinar tree. J Biomech Eng. 2009;131:1–15.
Lambert AR, O’Shaughnessy PT, Tawhai MH, et al. Regional deposition of particles in an image-based airway model: large-eddy simulation and left-right lung ventilation asymmetry. Aerosol science and technology. Aerosol Sci Technol. 2011;45:11–25.
Inthavong K, Choi L-T, Tu J, et al. Micron particle deposition in a tracheobronchial airway model under different breathing conditions. Med Eng Phys. 2010;32:1198–212.
Choi J, Tawhai M, Hoffman EA, et al. On intra- and intersubject variabilities of airflow in the human lungs. Phys Fluids. 2009;21:1017.
Li Z, Kleinstreuer CZZP. Particle deposition in the human tracheobronchial airways due to transient inspiratory flow patterns. Aerosol Science. 2007;38:625–44.
Lin C-L, Tawhai MH, McLennan G, et al. Characteristics of the turbulent laryngeal jet and its effect on airflow in the human intra-thoracic airways. Respir Physiol Neurobiol. 2007;157:295–309.
Tian G, Longest PW, Li XHM. Targeting aerosol deposition to and within the lung airways using excipient enhanced growth. J Aerosol Med Pulm Drug Deliv. 2013;26:248–65.
Tian G, Longest PW, Su GHM. Characterization of respiratory drug delivery with enhanced condensational growth (ECG) using an individual path model of the entire tracheobronchial airways. Ann Biomed Eng. 2011;39:1136–53.
Tian G, Longest PW, Su G, Walenga RLHM. Development of a stochastic individual path (SIP) model for predicting the tracheobronchial deposition of pharmaceutical aerosols: Effects of transient inhalation and sampling the airways. J Aerosol Sci. 2011;42:781–99.
Sosnowski TR. Powder particles and technologies for medicine delivery to the respiratory system: challenges and opportunities. KONA Powder Part J. 2018;2018(35):122–38.
Longest PW, Hindle M, Das Choudhuri SXJ. Comparison of ambient and spray aerosol deposition in a standard induction port and more realistic mouth-throat geometry. J Aerosol Sci. 2008;39:572–91.
Balashazy IHW. Particle deposition in airway bifurcations-II. Expiratory flow. J Aerosol Sci. 1993; 24:773–86.
Longest PWHM. Evaluation of the Respimat Soft Mist inhaler using a concurrent CFD and in vitro approach. J Aerosol Med Pulm Drug Deliv. 2009;22:99–112.
Longest PW, Tian G, Delvadia RHM. Development of a stochastic individual path (SIP) model for predicting the deposition of pharmaceutical aerosols: Effects of turbulence, polydisperse aerosol size, and evaluation of multiple lung lobes. Aerosol Sci Technol. 2012;46:1271–85.
Oldham MJ, Phalen RFHT. Computational fluid dynamic predictions and experimental results for particle deposition in an airway model. Aerosol Sci Technol. 2000;62:61–71.
Sznitman J, Sutter R, Altorfer D, Stampanonim M, Rosgen TSJ. Visualization of respiratory flows from 3D reconstructed alveolar airsapces using X-ray tomographic microscopy. J Vis. 2010;13:337–45.
Pw L. Numerical predictions of submicrometer aerosol deposition in the nasal cavity using a novel drift flux approach. Int J Heat Mass Transf. 2008;51:5562–77.
Golshahi L, Noga MLFW. Deposition of inhaled micrometer-sized particles in oropharyngeal airway replicas of children at constant flow rates. J Aerosol Sci. 2012;49:21–31.
Finlay W, Stapleton KYJ. On the use of computational fluid dynamics for simulating flow and particle deposition in the human respiratory tract. J Aerosol Med. 1996;9:329–41.
Kimbell JS, Gross EA, Joyner DR, et al. Application of computational fluid dynamics regional dosimetry of inhaled chemicals in the upper respiratory tract of the rat. Toxicol Appl Pharmacol. 1993;121:253–63.
Martonen TBGXF. Effects of tumors on inhaled pharmacologic drugs II. Particle motion. Cell Biochem Biophys. 2001;35:245–53.
Comer JK, Kleinstreuer C, Hyun S, et al. Aerosol transport and deposition in sequentially bifurcating airways. J Biomech Eng. 2000;4(122):152–8.
Ruzycki CA, Javaheri E, Finlay WH. The use of computational fluid dynamics in inhaler design. Expert Opin Drug Deliv. 2013;10:307–23.
Sheiner LB, Rosenberg BMV. Estimation of population characteristics of pharmacokinetic parameters from routine clinical data. J Pharmacokinet Biopharm. 1977;5:445–79.
Sheiner LB, Rosenberg BMK. Modelling of individual pharmacokinetics for computer-aided drug dosage. Comput Biomed Res. 1972;5:411–59.
Maier G, Rubino C, Hsu R, Grasela T, Baumgartner RA. Population pharmacokinetics of (R)-albuterol and (S)-albuterol in pediatric patients aged 4–11 years with asthma. Pulm Pharmacol Ther. 2007;20(5):534–42.
Minto C, Li B, Tattam B, Brown K, Seale JP, Donnelly R. Pharmacokinetics of epimeric budesonide and fluticasone propionate after repeat dose inhalation - intersubject variability in systemic absorption from the lung. Br J Clin Pharmacol. 2000;50:116–24.
Lönnebo A, Grahnén A, Karlsson MO. An integrated model for the effect of budesonide on ACTH and cortisol in healthy volunteers. Br J Clin Pharmacol. 2007;64(2):125–32.
Wu K, Goyal N, Stark JG, Hochhaus G. Evaluation of the administration time effect on the cumulative cortisol suppression and cumulative lymphocytes suppression for once-daily inhaled corticosteroids: A population modeling/simulation approach. J Clin Pharmacol. 2008;48(9):1069–80.
Soulele K, Macheras P, Karalis V. On the pharmacokinetics of two inhaled budesonide/formoterol combinations in asthma patients using modeling approaches. Pulm Pharmacol Ther. 2018;48:168–78.
Rohatagi S, Arya V, Zech K, Nave R, Hochhaus G, Jensen BK, et al. Population pharmacokinetics and pharmacodynamics of ciclesonide. J Clin Pharmacol. 2003;43(4):365–78.
Rohatagi S, Krishnaswami S, Pfister M, Sahasranaman S. Model-based covariate pharmacokinetic analysis and lack of cortisol suppression by the new inhaled corticosteroid ciclesonide using a novel cortisol release model. Am J Ther. 2005;12(5):385–97.
Xu J, Nave R, Lahu G, Derom E, Derendorf H. Population pharmacokinetics and pharmacodynamics of inhaled ciclesonide and fluticasone propionate in patients with persistent asthma. J Clin Pharmacol. 2010;50(10):1118–27.
Krishnaswami S, Hochhaus G, Möllmann H, Barth J, Derendorf H. Interpretation of absorption rate data for inhaled fluticasone propionate obtained in compartmental pharmacokinetic modeling. Int J Clin Pharmacol Ther. 2005;43(3):117–22.
Siederer S, Allen A, Yang S. Population Pharmacokinetics of Inhaled Fluticasone Furoate and Vilanterol in Subjects with Chronic Obstructive Pulmonary Disease. Eur J Drug Metab Pharmacokinet. 2016;41(6):743–58.
Soulele K, Macheras P, Silvestro L, Savu SR, Karalis V, Rizea Savu S, et al. Population Pharmacokinetics of Fluticasone Propionate/Salmeterol uisng two different dry powder inhalers. Eur J Pharm Sci. 2015;80:33–42.
Mehta R, Pefani E, Beerahee M, Brealey N, Barnacle H, Birk R, et al. Population Pharmacokinetic Analysis of Fluticasone Furoate/Umeclidinium/Vilanterol via a Single Inhaler in Patients with COPD. J Clin Pharmacol. 2018;58(11):1461–7.
Mehta R, Farrell C, Hayes S, Birk R, Okour M, Lipson DA. Population Pharmacokinetic Analysis of Fluticasone Furoate/Umeclidinium Bromide/Vilanterol in Patients with Chronic Obstructive Pulmonary Disease. Clin Pharmacokinet. 2020;59(1):67–79.
Bartels C, Looby M, Sechaud R, Kaiser G. Determination of the pharmacokinetics of glycopyrronium in the lung using a population pharmacokinetic modelling approach. Br J Clin Pharmacol. 2013;76(6):868–79.
Ambery CL, Wielders P, Ludwig-Sengpiel A, Chan R, Riley JH. Population Pharmacokinetics and Pharmacodynamics of GSK961081 (Batefenterol), a Muscarinic Antagonist and β2-Agonist, in Moderate-to-Severe COPD Patients: Substudy of a Randomized Trial. Drugs R D. 2015;15(3):281–91.
Demin I, Bartels C, Graham G, Bieth B, Gautier A, Tillmann HC, et al. Population pharmacokinetics of IND/GLY (indacaterol/glycopyrronium) in COPD patients. Int J Clin Pharmacol Ther. 2016;54(6):405–15.
Borghardt JM, Weber B, Staab A, Kunz C, Formella S, Kloft C. Investigating pulmonary and systemic pharmacokinetics of inhaled olodaterol in healthy volunteers using a population pharmacokinetic approach. Br J Clin Pharmacol. 2016;81(3):538–52.
Yang S, Lee L, Pascoe S. Population Pharmacokinetics Modeling of inhaled umeclidinium for adult patients with asthma. Eur J Drug Metab Pharmacokinet. 2016;42(1):https://doi.org/10.1007/s13318-016-0331-8.
Goyal N, Beerahee M, Kalberg C, Church A, Kilbride S, Mehta R. Population pharmacokinetics of inhaled umeclidinium and vilanterol in patients with chronic obstructive pulmonary disease. Clin Pharmacokinet. 2014;53(7):637–48.
Parra-Guillen ZP, Weber B, Sharma A, Freijer J, Retlich S, Borghardt JM, et al. Population Pharmacokinetic Analysis of Tiotropium in Healthy Volunteers after Intravenous Administration and Inhalation. J Pharmacokinet Pharmacodyn. 2014;41 Suppl 1:S54. Available from: https://www.researchgate.net/publication/270396120_Population_Pharmacokinetic_Analysis_of_Tiotropium_in_Healthy_Volunteers_after_Intravenous_Administration_and_Inhalation Accessed on 19 Nov 2020
FDA. Guidance for industry: population pharmacokinetics. US Department of Health and Human Services; Food and Drug Administration; Centre for Drug Evaluation and Research & Centre for Biologics Evaluation and Research. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/population-pharmacokinetics Accessed on 19 Nov 2020
Ette EI, Williams PJ. Population pharmacokinetics I: Background, concepts, and models. Ann Pharmacother. 2004;38(10):1702–6.
Shen DLZ. Population pharmacokinetics studies with nonlinear mixed effects modeling. Available from: support.sas.com/resources/papers/proceedings/proceedings/forum2007/148–2007.pdf.
Kim TH, Shin S, Shin BS. Model-based drug development: application of modeling and simulation in drug development. J Pharm Investig. 2018;48(4):431–41.
Dartois C, Brendel K, Comets E, Laffont CM, Laveille C, Tranchand B, et al. Overview of model-building strategies in population PK/PD analyses: 2002–2004 Literature survey. Br J Clin Pharmacol. 2007;64(5):603–12.
Lavé T, Parrott N, Grimm HP, Fleury A, Reddy M. Challenges and opportunities with modelling and simulation in drug discovery and drug development. Xenobiotica. 2007;37(10–11):1295–310.
Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol. 2011;51:45–73. Accessed on 19 Nov 2020
Lionberger R. New tools for generic orally inhaled drug products to maximize prospects of food and drug administration approval. Respir Drug Deliv. 2018;1:221–30.
Weber B, Hochhaus G. A pharmacokinetic simulation tool for inhaled corticosteroids. AAPS J. 2013;15(1):159–71.
Edginton AN, Joshi G. Have physiologically-based pharmacokinetic models delivered? Expert Opin Drug Metab Toxicol. 2011;7:929–34.
Jones HM. Physiologically based pharmacokinetic modelling in drug discovery and development: a pharmaceutical industry perspective. Clin Pharmacol Ther. 2015;97(3):247–62.
Chaudhuri SR, Lukacova V, Woltosz WS. Simulating the disposition of budesonide from dry powder inhalers (DPIs) and nebulizers. 2013; Available from: https://www.simulations-plus.com/assets/Chaudhuri-Modeling_Disp_Budesonide_IV_Oral_Admin_Healthy_Adult_Subjects-AAPS-2013.pdf Accessed on 19 Nov 2020
Caniga M, Cabal A, Mehta K, Ross DS, Gil MA, Woodhouse JD, et al. Preclinical experimental and mathematical approaches for assessing effective doses of inhaled drugs, using mometasone to support human dose predictions. J Aerosol Med Pulm Drug Deliv. 2016;29(4):362–77.
Boger E, Evans N, Chappell M, Lundqvist A, Ewing P, Wigenborg A, et al. Systems pharmacology approach for prediction of pulmonary and systemic pharmacokinetics and receptor occupancy of inhaled drugs. CPT: Pharmacomet Syst Pharmacol. 2016;5(4):201–10.
Boger E, Wigström O. A partial differential equation approach to inhalation physiologically based pharmacokinetic modeling. CPT: Pharmacomet Syst Pharmacol. 2018;7:638–46.
Boger E, Fridén M. Physiologically based pharmacokinetic/pharmacodynamic modeling accurately predicts the better bronchodilatory effect of inhaled versus oral salbutamol dosage forms. J Aerosol Med Pulm Drug Deliv. 2019;32(1):1–12.
Weber B, Hochhaus G. A systematic analysis of the sensitivity of plasma pharmacokinetics to detect differences in the pulmonary performance of inhaled fluticasone propionate products using a Model-Based Simulation Approach. AAPS J. 2015;17(4):999–1010.
Higashimori M, Ishikawa K, Gillen M, Zhou D. Physiologically based pharmacokinetic modelling of glycopyrronium in patients with renal impairment. J Pharm Sci. https://doi.org/10.1016/j.xphs.2020.03.014
Huang F, Zhu Q, Zhou X, Gou D, Yu J, Li R, et al. Role of CFD based in silico modelling in establishing an in vitro in vivo correlation of aerosol deposition in the respiratory tract. Adv Drug Deliv Rev. https://doi.org/10.1016/j.addr.2020.09.007.
Acknowledgements
The authors humbly thank Megha Kale (Librarian, Cipla Ltd) for immense support in referencing literature.
Author information
Authors and Affiliations
Contributions
Juliet Rebello was responsible for the conducting searches as per the search strategy, collecting the data and summarizing it. Dr. Sharvari Shukla reviewed the information. Dr. Bill Brashier helped improve the clarity of the manuscript in presentation and reviewed the technical content. He was employed at Cipla Ltd at the time of submission of this manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rebello, J., Brashier, B. & Shukla, S. Assessment of the predictive capability of modelling and simulation to determine bioequivalence of inhaled drugs: A systematic review. DARU J Pharm Sci 30, 229–243 (2022). https://doi.org/10.1007/s40199-021-00423-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-021-00423-7