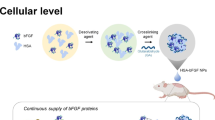
Abstract
Background
Developing an alternative and efficient therapy for wound healing has been an important research topic for pharmaceutical sciences. A straightforward but effective system for delivering fibroblast growth factor-2 (FGF-2) encoding plasmid DNA (pFGF-2) for wound healing therapy was aimed to develop in this study.
Methods
In order to provide the delivery of pFGF-2, a delivery vector, namely, cationic lipid nanoparticle (cLN) was developed by the melt-emulsification process, complexed with pFGF-2 to form a lipoplex system and further characterized. The pFGF-2 binding and protecting ability of lipoplexes were evaluated. The cytotoxicity and transfection efficiency of the lipoplexes, FGF-2 expression levels, and in vitro wound healing ability have been investigated on the L929 fibroblast cell line.
Results
The obtained lipoplex system has a particle size of 88.53 nm with a low PDI (0.185), and zeta potential values of 27.8 mV with a spherical shape. The ability of cLNs to bind pFGF-2 and protect against nucleases was demonstrated by gel retardation assay. Furthermore, the developed FGF-2 carrying lipoplexes system showed significant transfection and FGF-2 expression ability comparing naked plasmid. Finally, scratch assay revealed that the developed system is able to promote in vitro cell proliferation/migration in 48 h.
Conclusion
Promising results have been achieved with the use of lipoplexes carrying pFGF-2, and this approach could be considered as a potentially applicable concept for the future gene-based wound healing therapies.
Graphical abstract











Similar content being viewed by others
Availability of data and material
All data produced during the current study are available from the corresponding author on reasonable request.
References
Veith AP, Henderson K, Spencer A, Sligar AD, Baker AB. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Deliv Rev. 2019;146:97–125. https://doi.org/10.1016/j.addr.2018.09.010.
Pan A, Zhong M, Wu H, Peng Y, Xia H, Tang Q, et al. Topical Application of Keratinocyte Growth Factor Conjugated Gold Nanoparticles Accelerate Wound Healing. Nanomedicine Nanotechnology, Biol Med. 2018;14:1619–28. https://doi.org/10.1016/j.nano.2018.04.007.
Rajendran NK, Kumar SSD, Houreld NN, Abrahamse H. A review on nanoparticle based treatment for wound healing. J Drug Deliv Sci Technol. 2018;44:421–30. https://doi.org/10.1016/j.jddst.2018.01.009.
Laiva AL, O’Brien FJ, Keogh MB. Innovations in gene and growth factor delivery systems for diabetic wound healing. J Tissue Eng Regen Med. 2018;12:e296-312. https://doi.org/10.1002/term.2443.
Yoo Y, Hyun H, Yoon SJ, Kim SY, Lee DW, Um S, et al. Visible light-cured glycol chitosan hydrogel dressing containing endothelial growth factor and basic fibroblast growth factor accelerates wound healing in vivo. J Ind Eng Chem. 2018;67:365–72. https://doi.org/10.1016/j.jiec.2018.07.009.
Koike Y, Yozaki M, Utani A, Murota H. Fibroblast growth factor 2 accelerates the epithelial–mesenchymal transition in keratinocytes during wound healing process. Sci Rep. 2020;10:1–13. https://doi.org/10.1038/s41598-020-75584-7.
Akbaba H, Erel Akbaba G, Kantarcı AG. Development and evaluation of antisense shRNA-encoding plasmid loaded solid lipid nanoparticles against 5-α reductase activity. J Drug Deliv Sci Technol. 2018;44:270–7. https://doi.org/10.1016/J.JDDST.2018.01.001.
Fernandez-Piñeiro I, Pensado A, Badiola I, Sanchez A. Development and characterisation of chondroitin sulfate- and hyaluronic acid-incorporated sorbitan ester nanoparticles as gene delivery systems. Eur J Pharm Biopharm. 2018;125:85–94. https://doi.org/10.1016/j.ejpb.2018.01.009.
Şalva E, Akbuğa J. The Effects to GM-CSF Expression and Fibroblast Proliferation of pGMCSF Containing Chitosan/PVP Hydrogels. Marmara Pharm J. 2016;21:228–34. https://doi.org/10.12991/marupj.278854
Vij M, Natarajan P, Pattnaik BR, Alam S, Gupta N, Santhiya D, et al. Non-invasive topical delivery of plasmid DNA to the skin using a peptide carrier. J Control Release. 2016;222:159–68. https://doi.org/10.1016/j.jconrel.2015.12.017.
Vakilinezhad MA, Amini A, Akbari Javar H, Baha’addini Beigi Zarandi BF, Montaseri H, Dinarvand R. Nicotinamide loaded functionalized solid lipid nanoparticles improves cognition in Alzheimer’s disease animal model by reducing Tau hyperphosphorylation. Daru J Pharm Sci. 2018;26:165–77. https://doi.org/10.1007/s40199-018-0221-5.
Huang J, Wu L, Tashiro SI, Onodera S, Ikejima T. Fibroblast growth factor-2 suppresses oridonin-induced L929 apoptosis through extracellular signal-regulated kinase-dependent and phosphatidylinositol 3-kinase-independent pathway. J Pharmacol Sci. 2006;102:305–13. https://doi.org/10.1074/jbc.M109006200.
del Pozo-Rodríguez A, Pujals S, Delgado D, Solinís MA, Gascón AR, Giralt E, et al. A proline-rich peptide improves cell transfection of solid lipid nanoparticle-based non-viral vectors. J Control Release. 2009;133:52–9. https://doi.org/10.1016/j.jconrel.2008.09.004.
Erel-Akbaba G, İsar S, Akbaba H. Development and Evaluation of Solid Witepsol Nanoparticles for Gene Delivery. Turkish J Pharm Sci. 2021;18:344–51. https://doi.org/10.4274/tjps.galenos.2020.68878.
Erel-Akbaba G, Akbaba H, Kantarcı AG. Development and in vitro evaluation of positive-charged solid lipid nanoparticles as nucleic acid delivery system in glioblastoma treatment. Marmara Pharm J. 2018;22:299–306. https://doi.org/10.12991/mpj.2018.67.
Huang Y, Chen Z, Chen Y, Zhang H, Zhang Y, Zhao Y, et al. Effects of conformational alteration induced by d -/ l -isonucleoside incorporation in siRNA on their stability in serum and silencing activity. Bioconjug Chem. 2013;24:951–9. https://doi.org/10.1021/bc300642u.
Gaspar DP, Serra C, Lino PR, Goncalves L, Taboada P, Remunan-Lopez C, et al. Microencapsulated SLN: An innovative strategy for pulmonary protein delivery. Int J Pharm. 2017;516:231–46. https://doi.org/10.1016/j.ijpharm.2016.11.037.
Akbaba H, Erel-Akbaba G, Senturk S. Special Focus Issue Part II: Recruitment of solid lipid nanoparticles for the delivery of CRISPR/Cas9: primary evaluation of anticancer gene editing. Nanomedicine. 2021;16:963–78. https://doi.org/10.2217/nnm-2020-0412.
Paidikondala M, Kadekar S, Varghese OP. Innovative strategy for 3D transfection of primary human stem cells with BMP-2 expressing plasmid DNA: A clinically translatable strategy for ex vivo gene therapy. Int J Mol Sci. 2019;20. https://doi.org/10.3390/ijms20010056.
Teng J, Da Hora CC, Kantar RS, Nakano I, Wakimoto H, Batchelor TT, et al. Dissecting inherent intratumor heterogeneity in patient-derived glioblastoma culture models. Neuro Oncol. 2017;19:820–32. https://doi.org/10.1093/neuonc/now253.
Iannitti T, Scarrott JM, Likhite S, Coldicott IRP, Lewis KE, Heath PR, et al. Translating SOD1 gene silencing towards the clinic: A highly efficacious, off-target free and biomarker-supported strategy for familial ALS. Mol Ther - Nucleic Acids. 2018;12:75–88. https://doi.org/10.1016/j.omtn.2018.04.015.
Rameshk M, Sharififar F, Mehrabani M, Pardakhty A, Farsinejad A, Mehrabani M. Proliferation and In Vitro Wound Healing Effects of the Microniosomes Containing Narcissus tazetta L. Bulb Extract on Primary Human Fibroblasts (HDFs). DARU, J Pharm Sci.; 2018;26:31–42. https://doi.org/10.1007/s40199-018-0211-7.
Balasubramaniam MP, Murugan P, Chenthamara D, Ramakrishnan SG, Salim A, Lin FH, et al. Synthesis of chitosan-ferulic acid conjugated poly(vinyl alcohol) polymer film for an improved wound healing. Mater Today Commun. 2020;25: 101510. https://doi.org/10.1016/j.mtcomm.2020.101510.
Alwattar JK, Chouaib R, Khalil A, Mehanna MM. A novel multifaceted approach for wound healing: Optimization and in vivo evaluation of spray dried tadalafil loaded pro-nanoliposomal powder. Int J Pharm. 2020;587: 119647. https://doi.org/10.1016/j.ijpharm.2020.119647.
Kumari M, Liu C-H, Wu W-C. Efficient gene delivery by oligochitosan conjugated serum albumin: Facile synthesis, polyplex stability, and transfection. Carbohydr Polym. 2018; 148:37–49. https://www.sciencedirect.com/science/article/pii/S0144861717312894.
de Jesus MB, Zuhorn IS. Solid lipid nanoparticles as nucleic acid delivery system: Properties and molecular mechanisms. J Control Release. 2015;201:1–13. https://doi.org/10.1016/j.jconrel.2015.01.010.
Elsana H, Olusanya TOB, Carr-wilkinson J, Darby S, Faheem A, Elkordy AA. Evaluation of novel cationic gene based liposomes with cyclodextrin prepared by thin film hydration and microfluidic systems. Sci Rep. 2019;9:1–17. https://doi.org/10.1038/s41598-019-51065-4.
Jacobs C, Müller RH. Production and characterization of a budesonide nanosuspension for pulmonary administration. Pharm Res. 2002;19:189–94.
Vighi E, Ruozi B, Montanari M, Battini R, Leo E. Re-dispersible cationic solid lipid nanoparticles (SLNs) freeze-dried without cryoprotectors: Characterization and ability to bind the pEGFP-plasmid. Eur J Pharm Biopharm. 2007;67:320–8.
Thatipamula RP, Palem CR, Gannu R, Mudragada S, Yamsani MR. Formulation and in vitro characterization of domperidone loaded solid lipid nanoparticles and nanostructured lipid carriers. DARU, J Pharm Sci. 2011;19:23–32.
Wang MO, Etheridge JM, Thompson JA, Vorwald CE, Dean D, Fisher JP. Evaluation of the in vitro cytotoxicity of cross-linked biomaterials. Biomacromol. 2013;14:1321–9. https://doi.org/10.1021/bm301962f.
Argenziano M, Banche G, Luganini A, Finesso N, Allizond V, Gulino GR, et al. Vancomycin-loaded nanobubbles: A new platform for controlled antibiotic delivery against methicillin-resistant Staphylococcus aureus infections. Int J Pharm. 2017;523:176–88. https://doi.org/10.1016/j.ijpharm.2017.03.033.
Costa MF, Durço AO, Rabelo TK, Barreto R de SS, Guimarães AG. Effects of Carvacrol, Thymol and essential oils containing such monoterpenes on wound healing: a systematic review. J Pharm Pharmacol. 2019;71:141–55. https://doi.org/10.1111/jphp.13054.
Bellefroid C, Lechanteur A, Evrard B, Piel G. Lipid gene nanocarriers for the treatment of skin diseases: Current state-of-the-art. Eur J Pharm Biopharm. 2019;137:95–111. https://doi.org/10.1016/j.ejpb.2019.02.012.
Hu X, Qin N, Xue J, Li S, Huang X, Sun J, et al. Dehydrodiconiferyl alcohol from Silybum marianum (L.) Gaertn accelerates wound healing via inactivating NF-κB pathways in macrophages. J Pharm Pharmacol. 2020;72:305–17. https://doi.org/10.1111/jphp.13205.
Wu F, Dai L, Geng L, Zhu H, Jin T. Practically feasible production of sustained-release microspheres of granulocyte-macrophage colony-stimulating factor (rhGM-CSF). J Control Release. 2017;259:195–202. https://doi.org/10.1016/j.jconrel.2017.04.004.
Chopra S, Ruzgys P, Maciulevičius M, Jakutavičiute M, Šatka S. Investigation of plasmid DNA delivery and cell viability dynamics for optimal cell electrotransfection in vitro. Appl Sci. 2020;10(17):6070. https://doi.org/10.3390/app10176070.
Wang P, Huang S, Hu Z, Yang W, Lan Y, Zhu J, et al. In situ formed anti-inflammatory hydrogel loading plasmid DNA encoding VEGF for burn wound healing. Acta Biomater. 2019;100:191–201. https://doi.org/10.1016/j.actbio.2019.10.004.
Foldvari M, Chen DW, Nafissi N, Calderon D, Narsineni L, Rafiee A. Non-viral gene therapy: Gains and challenges of noninvasive administration methods. J Control Release . 2016;240:165–90. https://doi.org/10.1016/j.jconrel.2015.12.012.
Resnier P, David S, Lautram N, Delcroix GJR, Clavreul A, Benoit JP, et al. EGFR siRNA lipid nanocapsules efficiently transfect glioma cells in vitro. Int J Pharm. 2013;454:748–55. https://doi.org/10.1016/j.ijpharm.2013.04.001.
Karamanidou T, Karidi K, Bourganis V, Kontonikola K, Kammona O, Kiparissides C. Effective incorporation of insulin in mucus permeating self-nanoemulsifying drug delivery systems. Eur J Pharm Biopharm. 2018;183:37–49. https://doi.org/10.1016/j.carbpol.2017.11.013.
Ponti F, Campolungo M, Melchiori C, Bono N, Candiani G. Cationic lipids for gene delivery: many players, one goal. Chem Phys Lipids. 2021;235: 105032. https://doi.org/10.1016/j.chemphyslip.2020.105032.
Gooding M, Malhotra M, McCarthy DJ, Godinho BMDC, Cryan JF, Darcy R, et al. Synthesis and characterization of rabies virus glycoprotein-tagged amphiphilic cyclodextrins for siRNA delivery in human glioblastoma cells: in-vitro analysis. Eur J Pharm Sci. 2015;71:80–92. https://doi.org/10.1016/j.ejps.2015.02.007.
Qin Y, Tian Y, Liu Y, Li D, Zhang H, Yang Y, et al. Hyaluronic acid-modified cationic niosomes for ocular gene delivery: improving transfection efficiency in retinal pigment epithelium. J Pharm Pharmacol. 2018;70:1139–51. https://doi.org/10.1111/jphp.12940.
Bi YZ, Zhang YF, Cui CY, Ren LL, Jiang XY. Gene-silencing effects of anti-survivin siRNA delivered by RGDV-functionalized nanodiamond carrier in the breast carcinoma cell line MCF-7. Int J Nanomedicine. 2016;11:5771–87. https://doi.org/10.2147/IJN.S117611.
Zarei H, Kazemi Oskuee R, Hanafi-Bojd MY, Gholami L, Ansari L, Malaekeh-Nikouei B. Enhanced gene delivery by polyethyleneimine coated mesoporous silica nanoparticles. Pharm Dev Technol. 2019;24:127–32. https://doi.org/10.1080/10837450.2018.1431930.
Acknowledgements
The authors would like to thank Ayse Nalbantsoy and Batuhan Orman from the Ege University, Faculty of Engineering, Bioengineering Department, Izmir, Turkey for their help with flow cytometry experiments.
Funding
This project was supported by Izmir Katip Celebi University, Scientific Research Projects Coordinatorship (Project Number: 2019-ÖNAP-ECZF-0002).
Author information
Authors and Affiliations
Contributions
All authors contributed to this work presented in this manuscript. All authors have given approval for the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Erel-Akbaba, G., Akbaba, H. Investigation of the potential therapeutic effect of cationic lipoplex mediated fibroblast growth factor-2 encoding plasmid DNA delivery on wound healing. DARU J Pharm Sci 29, 329–340 (2021). https://doi.org/10.1007/s40199-021-00410-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-021-00410-y