Abstract
Background
Increasing bacterial resistance to quinolones is concerning. Hence, the development of novel quinolones by chemical modifications to overcome quinolone resistance is an attractive perspective in this context.
Objective
In this study, it is aimed to design and synthesize a novel series of functionalized fluoroquinolones using ciprofloxacin and sarafloxacin cores by hybridization of quinazolinone derivatives. This objective was tested by a comprehensive set of in vitro antibacterial assays in addition to SAR (structure–activity relationship) characterisation studies.
Methods
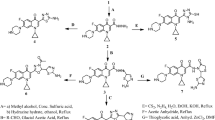
A nucleophilic reaction of ciprofloxacin and sarafloxacin with 2-(chloromethyl)quinazolin-4(3H)-one in the presence of NaHCO3 in dimethylformamide (DMF) was performed to obtain the desired compounds 5a-j. Novel compounds were characterised by 1H, 13C- NMR and IR spectroscopy, MS and elemental analysis. In silico pharmacokinetics prediction assays and molecular docking studies were performed to explore the binding characteristics and interactions. Antibacterial activities of the novel compounds were evaluated by Broth microdilution, well diffusion and disc diffusion assays against three gram-positive (Methicillin-resistant Staphylococcus aureus (MRSA), Staphylococcus aureus and Enterococcus faecalis) and three gram-negative bacteria (Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli).
Results
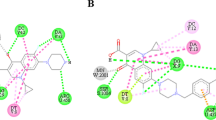
The compounds exhibited moderate to good activities against gram-positive bacteria and weak to moderate activities against gram-negative bacteria. Amongst all ciprofloxacin-derivatives, compound 5d was the most potent agent with high antibacterial activity against gram-positive bacteria, including MRSA and S. aureus ((minimum inhibitory concentration (MIC) = 16 nM for both), that is 60 times more potent than ciprofloxacin as parent drug. Compound 5i from sarafloxacin-derivatives was the most potent compound against MRSA and S. aureus (MIC = 0.125 μM). Well diffusion and disk diffusion assay results demonstrated confirmatory outcomes for the quantitative broth microdilution assay. Molecular docking study results were in accordance with the results of antibacterial activity assays.
Conclusion
The results of the current study demonstrated that the novel ciprofloxacin and sarafloxacin derivatives synthesized here have promising antibacterial activities. Particularly, compounds 5d and 5i have potential for wider antibacterial applications following further analysis.
Graphical abstract






Similar content being viewed by others
References
Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645–58.
McAdam AJ, Hooper DC, DeMaria A, Limbago BM, O'Brien TF, McCaughey B. Antibiotic resistance: how serious is the problem, and what can be done? Clin Chem. 2012;58:1182–6.
Chokshi A, Sifri Z, Cennimo D, Horng H. Global contributors to antibiotic resistance. J Global Infect Dis. 2019;11:36.
Chandler CI. Current accounts of antimicrobial resistance: stabilisation, individualisation and antibiotics as infrastructure. Palgrave Commun. 2019;5:1–13.
Chao Y-., Farrah K. Fluoroquinolones for the treatment of urinary tract infection: a review of clinical effectiveness, cost-effectiveness, and guidelines. 2019.
Zaffiri L, Gardner J, Toledo-Pereyra LH. History of antibiotics: from fluoroquinolones to daptomycin (part 2). J Investig Surg. 2013;26:167–79.
Gao F, Wang P, Yang H, Miao Q, Ma L, Lu G. Recent developments of quinolone-based derivatives and their activities against Escherichia coli. Eur J Med Chem. 2018;157:1223–48.
Pham TD, Ziora ZM, Blaskovich MA. Quinolone antibiotics. Medchemcomm. 2019;10:1719–39.
Aldred KJ, Kerns RJ, Osheroff N. Mechanism of quinolone action and resistance. Biochemistry. 2014;53:1565–74.
Andersson MI, MacGowan AP. Development of the quinolones. J Antimicrob Chemother. 2003;51:1–11.
Naeem A, Badshah SL, Muska M, Ahmad N, Khan K. The current case of quinolones: synthetic approaches and antibacterial activity. Molecules. 2016;21:268.
Drlica K, Hiasa H, Kerns R, Malik M, Mustaev A, Zhao X. Quinolones: action and resistance updated. Curr Top Med Chem. 2009;9:981–98.
Anderson VE, Osheroff N. Type II topoisomerases as targets for quinolone antibacterials turning Dr. Jekyll into Mr. Hyde. Curr Pharm Des. 2001;7:337–53.
Wohlkonig A, Chan PF, Fosberry AP, Homes P, Huang J, Kranz M, et al. Structural basis of quinolone inhibition of type IIA topoisomerases and target-mediated resistance. Nat Struct Mol Biol. 2010;17:1152–3.
Mitscher LA. Bacterial topoisomerase inhibitors: quinolone and pyridone antibacterial agents. Chem Rev. 2005;105:559–92.
Segatore B, Setacci D, Perilli M, Franceschini N, Marchetti F, Amicosante G. Bactericidal activity of levofloxacin and ciprofloxacin on clinical isolates of different phenotypes of Pseudomonas aeruginosa. Int J Antimicrob Agents. 2000;13:223–6.
Sriram D, Yogeeswari P, Basha JS, Radha DR, Nagaraja V. Synthesis and antimycobacterial evaluation of various 7-substituted ciprofloxacin derivatives. Bioorg Med Chem. 2005;13:5774–8.
Aldred KJ, McPherson SA, Wang P, Kerns RJ, Graves DE, Turnbough CL Jr, et al. Drug interactions with bacillus anthracis topoisomerase IV: biochemical basis for quinolone action and resistance. Biochemistry. 2012;51:370–81.
Aldred KJ, McPherson SA, Turnbough CL Jr, Kerns RJ, Osheroff N. Topoisomerase IV-quinolone interactions are mediated through a water-metal ion bridge: mechanistic basis of quinolone resistance. Nucleic Acids Res. 2013;41:4628–39.
Anderson VE, Zaniewski RP, Kaczmarek FS, Gootz TD, Osheroff N. Action of quinolones against Staphylococcus aureus topoisomerase IV: basis for DNA cleavage enhancement. Biochemistry. 2000;39:2726–32.
Barnard FM, Maxwell A. Interaction between DNA gyrase and quinolones: effects of alanine mutations at GyrA subunit residues Ser83and Asp87. Antimicrob Agents Chemother. 2001;45:1994–2000.
Peterson LR. Quinolone molecular structure-activity relationships: what we have learned about improving antimicrobial activity. Clin Infect Dis. 2001;33:S180–6.
Asadipour A, Moshafi MH, Khosravani L, Moghimi S, Amou E, Firoozpour L, et al. N-substituted piperazinyl sarafloxacin derivatives: synthesis and in vitro antibacterial evaluation. DARU J Pharm Sci. 2018;26:199–207.
Foroumadi A, Emami S, Mehni M, Moshafi MH, Shafiee A. Synthesis and antibacterial activity of N-[2-(5-bromothiophen-2-yl)-2-oxoethyl] and N-[(2-5-bromothiophen-2-yl)-2-oximinoethyl] derivatives of piperazinyl quinolones. Bioorg Med Chem Lett. 2005;15:4536–9.
Gatadi S, Lakshmi TV, Nanduri S. 4 (3H)-Quinazolinone derivatives: promising antibacterial drug leads. Eur J Med Chem. 2019;170:157–72.
Cao S-L, Feng Y-P, Jiang Y-Y, Liu S-Y, Ding G-Y, Li R-T. Synthesis and in vitro antitumor activity of 4 (3H)-quinazolinone derivatives with dithiocarbamate side chains. Bioorg Med Chem Lett. 2005;15:1915–7.
Giri RS, Thaker HM, Giordano T, Williams J, Rogers D, Sudersanam V, et al. Design, synthesis and characterization of novel 2-(2, 4-disubstituted-thiazole-5-yl)-3-aryl-3H-quinazoline-4-one derivatives as inhibitors of NF-κB and AP-1 mediated transcription activation and as potential anti-inflammatory agents. Eur J Med Chem. 2009;44:2184–9.
Jatav V, Mishra P, Kashaw S, Stables J. CNS depressant and anticonvulsant activities of some novel 3-[5-substituted 1, 3, 4-thiadiazole-2-yl]-2-styryl quinazoline-4 (3H)-ones. Eur J Med Chem. 2008;43:1945–54.
Xia Y, Yang Z-Y, Hour M-J, Kuo S-C, Xia P, Bastow KF, et al. Antitumor agents. Part 204: synthesis and biological evaluation of substituted 2-aryl quinazolinones. Bioorg Med Chem Lett. 2001;11:1193–6.
Jessy E, Sambanthan AT, Alex J, Sridevi C, Srinivasan K. Synthesis and biological evaluation of some novel quinazolones. Indian J Pharm Sci. 2007;69:476.
Alagarsamy V, Thangathiruppathy A, Rajasekaran S, Vijaykumar S, Revathi R, Anburaj J, et al. Pharmacological evaluation of 2-substituted (1, 3, 4) thiadiazolo quinazolines. Indian J Pharm Sci. 2006;68:108.
Reddy MM, Sivaramakrishna A. Remarkably flexible quinazolinones—synthesis and biological applications. J Heterocyclic Chem. 2020;57:942–54.
Kerns RJ, Rybak MJ, Kaatz GW, Vaka F, Cha R, Grucz RG, et al. Structural features of piperazinyl-linked ciprofloxacin dimers required for activity against drug-resistant strains of Staphylococcus aureus. Bioorg Med Chem Lett. 2003;13:2109–12.
Wang S, Jia X-D, Liu M-L, Lu Y, Guo H-Y. Synthesis, antimycobacterial and antibacterial activity of ciprofloxacin derivatives containing a N-substituted benzyl moiety. Bioorg Med Chem Lett. 2012;22:5971–5.
Akhtar R, Yousaf M, Naqvi SAR, Irfan M, Zahoor AF, Hussain AI, et al. Synthesis of ciprofloxacin-based compounds: a review. Synth Commun. 2016;46:1849–79.
Bauer A. Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol. 1966;45:149–58.
Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717.
O’Boyle N, Banck M, James C, Morley C, Vandermeersch T, Hutchison G. Open babel: an open chemical toolbox. J Cheminformatics. 2011;1:33.
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–91.
Khan SA, Saleem K, Khan Z. Synthesis, structure elucidation and antibacterial evaluation of new steroidal-5-en-7-thiazoloquinoxaline derivatives. Eur J Med Chem. 2008;43:2257–61.
Acknowledgments
This work was supported by grants from Research Council of Tehran University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2072 kb)
Rights and permissions
About this article
Cite this article
Norouzbahari, M., Salarinejad, S., Güran, M. et al. Design, synthesis, molecular docking study, and antibacterial evaluation of some new fluoroquinolone analogues bearing a quinazolinone moiety. DARU J Pharm Sci 28, 661–672 (2020). https://doi.org/10.1007/s40199-020-00373-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-020-00373-6