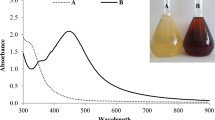
Abstract
Background
The purpose of the present study was to investigate the antioxidant and antimicrobial activities of a conventional preservative system containing desferrioxamine mesylate (DFO) and optimize the composition of the system through mathematical models.
Methods
Different combinations of ethylenediaminetetraacetic acid (EDTA), sodium metabisulfite (SM), DFO and methylparaben (MP) were prepared using factorial design of experiments. The systems were added to ascorbic acid (AA) solution and the AA content over time, at room temperature and at 40 °C was determined by volumetric assay. The systems were also evaluated for antioxidant activity by a fluorescence-based assay. Antimicrobial activity was assessed by microdilution technique and photometric detection against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Candida albicans and Aspergillus brasiliensis. A multi-criteria decision approach was adopted to optimize all responses by desirability functions.
Results
DFO did not extend the stability of AA over time, but displayed a better ability than EDTA to block the pro-oxidant activity of iron. DFO had a positive interaction with MP in microbial growth inhibition. The mathematical models showed adequate capacity to predict the responses. Statistical optimization aiming to meet the quality specifications of the ascorbic acid solution indicated that the presence of DFO in the composition allows to decrease the concentrations of EDTA, SM and MP.
Conclusion
DFO was much more effective than EDTA in preventing iron-catalyzed oxidation. In addition, DFO improved the inhibitory response of most microorganisms tested. The Quality by Design concepts aided in predicting an optimized preservative system with reduced levels of conventional antioxidants and preservatives, suggesting DFO as a candidate for multifunctional excipient.
Graphical abstract









Similar content being viewed by others
References
Yu LX, Amidon G, Khan MA, Hoag SW, Polli J, Raju GK, et al. Understanding pharmaceutical quality by design. AAPS J. 2014;16(4):771–83.
Halla N, Fernandes IP, Heleno SA, Costa P, Boucherit-Otmani Z, Boucheirt K, et al. Cosmetics preservation: a review on present strategies. Molecules. 2018;23(7):1–41.
Pifferi G, Santoro P, Pedrani M. Quality and functionality of excipients. Farmaco. 1999;54(1–2):1–14.
Abrutyn ES. Deciphering chelating agent formulas. In: Cosmetics & Toiletries. 2013. https://www.cosmeticsandtoiletries.com/formulating/function/aids/premium-deciphering-chelating-agent-formulas-215885521.html. Accessed 12 Nov 2019.
Chaturvedi S, Dave PN. Removal of iron for safe drinking water. Desalination. 2012;303:1–11.
Kikuchi T, Suzuki M, Kusai A, Iseki K, Sasaki H. Synergistic effect of EDTA and boric acid on corneal penetration of CS-088. Int J Pharm. 2005;290(1–2):83–9.
Aguilera A, Bermudez Y, Martínez E, Marrero MA, Muñoz L, Páez R, et al. Formulation development of a recombinant streptokinase suppository for hemorrhoids treatment. Biotecnol Apl. 2013;30(3):182–6.
Alakomi H-L, Paananen A, Suihko M-L, Helander IM, Saarela M. Weakening effect of cell permeabilizers on gram-negative bacteria causing biodeterioration. Appl Environ Microbiol. 2006;72(7):4695–703.
Herman A. Antimicrobial ingredients as preservative booster and components of self-preserving cosmetic products. Curr Microbiol 2018.
Varvaresou A, Papageorgiou S, Tsirivas E, Protopapa E, Kintziou H, Kefala V, et al. Self-preserving cosmetics. Int J Cosmet Sci. 2009;1(3):163–7.
Narayanan M, Sekar P, Pasupathi M, Mukhopadhyay T. Self-preserving personal care products. Int J Cosmet Sci. 2017;39(3):301–9.
Brovč EV, Pajk S, Šink R, Mravljak J. Protein formulations containing polysorbates: are metal chelators needed at all? Antioxidants (Basel). 2020;9(5):441.
Genuis SJ, Birkholz D, Curtis L, Sandau C. Paraben levels in an urban community of Western Canada. ISRN Toxicol. 2013.
Rajan JP, Cornell R, White AA. A case of systemic contact dermatitis secondary to edetate disodium. J Allergy Clin Immunol Pract. 2015;3(4):607–8.
Pruitt C, Warshaw EM. Allergic contact dermatitis from ethylenediaminetetraacetic acid. Dermatitis. 2010;21(2):121–2.
Laborde-Castérot H, Villa AF, Rosenberg N, Dupont P, Lee HM, Garnier R. Occupational rhinitis and asthma due to EDTA-containing detergents or disinfectants. Am J Ind Med. 2012;55(8):677–82.
Schmidt CK, Brauch HJ. Impact of aminopolycarboxylates on aquatic organisms and eutrophication: overview of available data. Environ Toxicol. 2004;19(6):620–37.
Yamamoto H, Tamura I, Hirata Y, Kato J, Kagota K, Katsuki S, et al. Aquatic toxicity and ecological risk assessment of seven parabens: Individual and additive approach. Sci Total Environ. 2011;410–411:102–11.
Haman C, Dauchy X, Rosin C, Munoz JF. Occurrence, fate and behavior of parabens in aquatic environments: a review. Water Res. 2015;68:1–11.
Oviedo C, Rodríguez J. EDTA: the chelating agent under environmental scrutiny. Quim Nova. 2003;26(6):901–5.
Byrne SL, Krishnamurthy D, Wessling-Resnick M. Pharmacology of iron transport. Annu Rev Pharmacol Toxicol. 2013;53:17–36.
Fleming RE, Ponka P. Iron overload in human disease. N Engl J Med. 2012;366(4):348–59.
Hider RC, Kong X. Chemistry and biology of siderophores. Nat Prod Rep. 2010;27(5):637–57.
Breuer W, Ermers MJ, Pootrakul P, Abramov A, Hershko C, Cabantchik ZI. Desferrioxamine-chelatable iron, a component of serum non-transferrin-bound iron, used for assessing chelation therapy. Blood. 2001;97(3):792–8.
Kontoghiorghes GJ, Kolnagou A, Skiada A, Petrikkos G. The role of iron and chelators on infections in iron overload and non iron loaded conditions: prospects for the design of new antimicrobial therapies. Hemoglobin. 2010;34(3):227–39.
Xu B, Kong XL, Zhou T, Qiu DH, Chen YL, Liu MS, et al. Synthesis, iron(III)-binding affinity and in vitro evaluation of 3-hydroxypyridin-4-one hexadentate ligands as potential antimicrobial agents. Bioorg Med Chem Lett. 2011;21(21):6376–80.
Holbein BE, Mira de Orduña R. Effect of trace iron levels and iron withdrawal by chelation on the growth of Candida albicans and Candida vini. FEMS Microbiol Lett. 2010;307(1):19–24.
Fukuda IM, Pinto CFF, Moreira CS, Saviano AM, Lourenço FR. Design of Experiments (DoE) applied to pharmaceutical and analytical quality by design (QbD). Braz J Pharm Sci. 2018;54(special issue):1–16.
Meka VS, Nali SR, Songa AS, Battu JR, Kolapalli VR. Statistical optimization of a novel excipient (CMEC) based gastro retentive floating tablets of propranolol HCl and it's in vivo buoyancy characterization in healthy human volunteers. Daru. 2012;20:21.
United States Pharmacopeia. 37th ed. Rockville: The United States Pharmacopeial Convention; 2017.
Zenebon O, Pascuet NS, Tiglea P. Métodos físico-químicos para análise de alimentos. São Paulo: Instituto Adolfo Lutz; 2008.
International Conference on Harmonisation. Validation of analytical procedures: text and methodology Q2(R1). Genebra: ICH; 2005.
Esposito BP, Breuer W, Sirankapracha P, Pootrakul P, Hershko C, Cabantchik ZI. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood. 2003;102(7):2670–7.
Wayne PA. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. CLSI standard M07.11th ed. Clinical and Laboratory Standards Institute; 2018.
Candioti LV, Zan MMD, Cámara MS, Goicoechea HC. Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta. 2014;124:123–38.
Stamford NPJ. Stability, transdermal penetration, and cutaneous effects of ascorbic acid and its derivatives. J Cosmet Dermatol. 2012;11(4):310–7.
Miller CJ, Rose AL, Waite TD. Importance of iron complexation for Fenton-mediated hydroxyl radical production at circumneutral pH. Front Mar Sci. 2016;3:1–13.
Ihnat PM, Vennerstrom JL, Robinson DH. Solution equilibria of deferoxamine amides. J Pharm Sci. 2002;91(7):1733–41.
Lundov MD, Moesby L, Zachariae C, Johansen JD. Contamination versus preservation of cosmetics: a review on legislation, usage, infections, and contact allergy. Contact Derm. 2009;60(2):70–8.
Deza G, Giménez-Arnau AM. Allergic contact dermatitis in preservatives: current standing and future options. Curr Opin Allergy Clin Immunol. 2017;17(4):263–8.
Polson G, Jourden J, Zheng Q, Prioli RM, Ciccognani D, Choi S; Arch Chemicals, lnc. (US). Biocidal compositions comprising iron chelators. Patent WO 2014/059417 A1. 2014 Apr 17.
Gokarn K, Pal RB. Activity of siderophores against drug-resistant gram-positive and gram-negative bacteria. Infect Drug Resist. 2018;11:61–75.
Thompson MG, Corey BW, Si Y, Craft DW, Zurawski DV. Antibacterial activities of iron chelators against common nosocomial pathogens. Antimicrob Agents Chemother. 2012;56(10):5419–21.
Lai YW, Campbell LT, Wilkins MR, Pang CN, Chen S, Carter DA. Synergy and antagonism between iron chelators and antifungal drugs in Cryptococcus. Int J Antimicrob Agents. 2016;48(4):388–94.
Huayhuaz JAA, Vitorino HA, Campos OS, Serrano SHP, Kaneko TM, Espósito BP. Desferrioxamine and desferrioxamine-caffeine as carriers of aluminum and gallium to microbes via the Trojan horse effect. J Trace Elem Med Biol. 2017;41:16–22.
Lourenço FR, Francisco FL, Ferreira MR, Andreoli T, Löbenberg R, Bou-Chacra N. Design space approach for preservative system optimization of an anti-aging eye fluid emulsion. J Pharm Pharm Sci. 2015;18(3):551–61.
Ferreira MRS, Lourenço FR, Ohara MT, Bou-Chacra NA, Pinto TJA. An innovative challenge test for solid cosmetics using freeze-dried microorganisms and electrical methods. J Microbiol Methods. 2014;106:104–9.
van Asbeck BS, Marcelis JH, van Kats JH, Jaarsma EY, Verhoef J. Synergy between the iron chelator deferoxamine and the antimicrobial agents gentamicin, chloramphenicol, cefalothin, cefotiam and cefsulodin. Eur J Clin Microbiol. 1983;2(5):432–8.
Hartzen SH, Frimodt-Møller N, Thomsen VF. The antibacterial activity of a siderophore. 3. The activity of deferoxamine in vitro and its influence on the effect of antibiotics against Escherichia coli, Proteus mirabilis and coagulase-negative staphylococci. APMIS. 1994;102(3):219–26.
Desai NP, Yang A, Ci SX, De T, Trieu V, Soon-Shiong P; American Biosciences, Inc. (US). Compositions and methods of delivery of pharmacological agents. Patent WO 2004/052401, 2004 jun 24.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lombardo, M., Espósito, B.P., Lourenço, F.R. et al. The application of pharmaceutical quality by design concepts to evaluate the antioxidant and antimicrobial properties of a preservative system including desferrioxamine. DARU J Pharm Sci 28, 635–646 (2020). https://doi.org/10.1007/s40199-020-00370-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-020-00370-9