Abstract
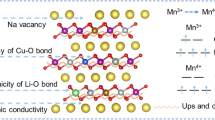
High-entropy oxides (HEOs) and medium-entropy oxides (MEOs) are new types of single-phase solid solution materials. MEOs have rarely been reported as positive electrode material for sodium-ion batteries (SIBs). In this study, we first proposed the concept of the application of MEOs in SIBs. P2-type 3-cation oxide Na2/3Ni1/3Mn1/3Fe1/3O2 (NaNMF) and 4-cation oxide Na2/3Ni1/3Mn1/3Fe1/3−xAlxO2 (NaNMFA) were prepared using the solid-state method, rather than the doping technology. In addition, the importance of the concept of entropy stabilization in material performance and battery cycling was demonstrated by testing 3-cation (NaNMF) and 4-cation (NaNMFA) oxides in the same system. Thus, NaNMFA can provide a reversible capacity of about 125.6 mAh·g−1 in the voltage range of 2–4.2 V, and has enhanced cycle stability. The capacity and decay law of the MEO batteries indicate that the configurational entropy (1.28 R (NaNMFA) > 1.10 R (NaNMF)) of the cationic system, is the main factor affecting the structural and cycle stability of the electrode material. This work emphasizes that the rational design of MEOs with novel structures and different electrochemically active elements may be the strategy for exploring high-performance SIB cathode materials in next-generation energy storage devices.

Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Yabuuchi N, Kubota K, Dahbi M, et al. Research development on sodium-ion batteries. Chem Rev 2014, 114: 11636–11682.
Yi TF, Pan JJ, Wei TT, et al. NiCo2S4-based nanocomposites for energy storage in supercapacitors and batteries. Nano Today 2020, 33: 100894.
Titirici MM. Sustainable batteries—quo vadis? Adv Energy Mater 2021, 11: 2003700.
Armand M, Tarascon JM. Building better batteries. Nature 2008, 451: 652–657.
Hwang JY, Myung ST, Sun YK. Sodium-ion batteries: Present and future. Chem Soc Rev 2017, 46: 3529–3614.
Yi TF, Wei TT, Li Y, et al. Efforts on enhancing the Li-ion diffusion coefficient and electronic conductivity of titanate-based anode materials for advanced Li-ion batteries. Energy Storage Mater 2020, 26: 165–197.
Hou HS, Banks CE, Jing MJ, et al. Carbon quantum dots and their derivative 3D porous carbon frameworks for sodium-ion batteries with ultralong cycle life. Adv Mater 2015, 27: 7861–7866.
Hou HS, Shao LD, Zhang Y, et al. Large-area carbon nanosheets doped with phosphorus: A high-performance anode material for sodium-ion batteries. Adv Sci 2017, 4: 1600243.
Hong WW, Zhang Y, Yang L, et al. Carbon quantum dot micelles tailored hollow carbon anode for fast potassium and sodium storage. Nano Energy 2019, 65: 104038.
Wu CJ, Hua WB, Zhang Z, et al. Design and synthesis of layered Na2Ti3O7 and tunnel Na2Ti6O13 hybrid structures with enhanced electrochemical behavior for sodium-ion batteries. Adv Sci 2018, 5: 1800519.
Xiao Y, Wang PF, Yin YX, et al. Exposing {010} active facets by multiple-layer oriented stacking nanosheets for high-performance capacitive sodium-ion oxide cathode. Adv Mater 2018, 30: 1803765.
Xiao Y, Zhu YF, Xiang W, et al. Deciphering an abnormal layered-tunnel heterostructure induced by chemical substitution for the sodium oxide cathode. Angew Chemie Int Ed 2020, 59: 1491–1495.
You Y, Manthiram A. Progress in high-voltage cathode materials for rechargeable sodium-ion batteries. Adv Energy Mater 2018, 8: 1701785.
Clément RJ, Bruce PG, Grey CP. Review—Manganese-based P2-type transition metal oxides as sodium-ion battery cathode materials. J Electrochem Soc 2015, 162: A2589–A2604.
Ma C, Alvarado J, Xu J, et al. Exploring oxygen activity in the high energy P2-type Na0.78Ni0.23Mn0.69O2 cathode material for Na-ion batteries. J Am Chem Soc 2017, 139: 4835–4845.
Maitra U, House RA, Somerville JW, et al. Oxygen redox chemistry without excess alkali-metal ions in Na2/3[Mg0.28Mn0.72]O2. Nat Chem 2018, 10: 288–295.
Rong XH, Liu J, Hu EY, et al. Structure-induced reversible anionic redox activity in Na layered oxide cathode. Joule 2018, 2: 125–140.
Zhao QQ, Butt FK, Guo ZF, et al. High-voltage P2-type manganese oxide cathode induced by titanium gradient modification for sodium ion batteries. Chem Eng J 2021, 403: 126308.
Wang PF, Yao HR, Liu XY, et al. Na+/vacancy disordering promises high-rate Na-ion batteries. Sci Adv 2018, 4: eaar6018.
Wang QC, Meng JK, Yue XY, et al. Tuning P2-structured cathode material by Na-site Mg substitution for Na-ion batteries. J Am Chem Soc 2019, 141: 840–848.
Wang PF, You Y, Yin YX, et al. Suppressing the P2-O2 phase transition of Na0.67Mn0.67Ni0.33O2 by magnesium substitution for improved sodium-ion batteries. Angewandte Chemie 2016, 128: 7571–7575.
Singh G, Tapia-Ruiz N, Lopez del Amo JM, et al. High voltage Mg-doped Na0.67Ni0.3−xMgxMn0.7O2 (x = 0.05, 0.1) Na-ion cathodes with enhanced stability and rate capability. Chem Mater 2016, 28: 5087–5094.
Huang Q, Liu JT, Xu S, et al. Roles of coherent interfaces on electrochemical performance of sodium layered oxide cathodes. Chem Mater 2018, 30: 4728–4737.
Ma C, Alvarado J, Xu J, et al. Exploring oxygen activity in the high energy P2-type Na0.78Ni0.23Mn0.69O2 cathode material for Na-ion batteries. J Am Chem Soc 2017, 139: 4835–4845.
Zheng LM, Wang ZQ, Wu MS, et al. Jahn-Teller type small polaron assisted Na diffusion in NaMnO2 as a cathode material for Na-ion batteries. J Mater Chem A 2019, 7: 6053–6061.
Hwang JY, Kim J, Yu TY, et al. A new P2-type layered oxide cathode with superior full-cell performances for K-ion batteries. J Mater Chem A 2019, 7: 21362–21370.
Kumakura S, Tahara Y, Kubota K, et al. Sodium and manganese stoichiometry of P2-type Na2/3MnO2. Angew Chem Int Ed 2016, 55: 12760–12763.
Wu XH, Guo JH, Wang DW, et al. P2-type Na0.66Ni0.33−xZnxMn0.67O2 as new high-voltage cathode materials for sodium-ion batteries. J Power Sources 2015, 281: 18–26.
You Y, Xin S, Asl HY, et al. Insights into the improved high-voltage performance of Li-incorporated layered oxide cathodes for sodium-ion batteries. Chem 2018, 4: 2124–2139.
Huang WN, Xing LD, Zhang RQ, et al. A novel electrolyte additive for improving the interfacial stability of high voltage lithium nickel manganese oxide cathode. J Power Sources 2015, 293: 71–77.
Put B, Vereecken PM, Labyedh N, et al. High cycling stability and extreme rate performance in nanoscaled LiMn2O4 thin films. ACS Appl Mater Interfaces 2015, 7: 22413–22420.
Zhao WW, Kirie H, Tanaka A, et al. Synthesis of metal ion substituted P2-Na2/3Ni1/3Mn2/3O2 cathode material with enhanced performance for Na ion batteries. Mater Lett 2014, 135: 131–134.
Hemalatha K, Jayakumar M, Bera P, et al. Improved electrochemical performance of Na0.67MnO2 through Ni and Mg substitution. J Mater Chem A 2015, 3: 20908–20912.
Wu XH, Guo JH, Wang DW, et al. P2-type Na0.66Ni0.33−xZnxMn0.67O2 as new high-voltage cathode materials for sodium-ion batteries. J Power Sources 2015, 281: 18–26.
Chung KY, Yoon WS, Lee HS, et al. Comparative studies between oxygen-deficient LiMn2O4 and Al-doped LiMn2O4. J Power Sources 2005, 146: 226–231.
Clément RJ, Billaud J, Robert Armstrong A, et al. Structurally stable Mg-doped P2-Na2/3Mn1−yMgyO2 sodium-ion battery cathodes with high rate performance: Insights from electrochemical, NMR and diffraction studies. Energy Environ Sci 2016, 9: 3240–3251.
Luo C, Langrock A, Fan XL, et al. P2-type transition metal oxides for high performance Na-ion battery cathodes. J Mater Chem A 2017, 5: 18214–18220.
Ramasamy HV, Kaliyappan K, Thangavel R, et al. Cu-doped P2-Na0.5Ni0.33Mn0.67O2 encapsulated with MgO as a novel high voltage cathode with enhanced Na-storage properties. J Mater Chem A 2017, 5: 8408–8415.
Bucher N, Hartung S, Franklin JB, et al. P2-NaxCoyMn1−yO2 (y = 0, 0.1) as cathode materials in sodium-ion batteries—Effects of doping and morphology to enhance cycling stability. Chem Mater 2016, 28: 2041–2051.
Kong WJ, Wang HB, Zhai YW, et al. Enhancing the rate capability and cycling stability of Na0.67Mn0.7Fe0.2Co0.1O2 through a synergy of Zr4+ doping and ZrO2 coating. J Phys Chem C 2018, 122: 25909–25916.
Pang WL, Zhang XH, Guo JZ, et al. P2-type Na2/3Mn1−xAlxO2 cathode material for sodium-ion batteries: Al-doped enhanced electrochemical properties and studies on the electrode kinetics. J Power Sources 2017, 356: 80–88.
Wu X, Xu GL, Zhong G, et al. Insights into the effects of zinc doping on structural phase transition of P2-type sodium nickel manganese oxide cathodes for high-energy sodium ion batteries. ACS Appl Mater Interfaces 2016, 8: 22227–22237.
Yuan DD, Hu XH, Qian JF, et al. P2-type Na0.67Mn0.65Fe0.2Ni0.15O2 cathode material with High-capacity for sodium-ion battery. Electrochimica Acta 2014, 116: 300–305.
Wang K, Wu ZG, Zhang T, et al. P2-type Na0.67Mn0.72Ni0.14Co0.14O2 with K+ doping as new high rate performance cathode material for sodium-ion batteries. Electrochimica Acta 2016, 216: 51–57.
Zhao CL, Ding FX, Lu YX, et al. High-entropy chemistry stabilizing layered O3-type structure in Na-ion cathode. Angew Chem Int Ed 2020, 59: 264–269.
Zhao ZF, Xiang HM, Chen H, et al. High-entropy (Nd0.2Sm0.2Eu0.2Y0.2Yb0.2)4Al2O9 with good high temperature stability, low thermal conductivity, and anisotropic thermal expansivity. J Adv Ceram 2020, 9: 595–605.
Wang QS, Sarkar A, Wang D, et al. Multi-anionic and -cationic compounds: New high entropy materials for advanced Li-ion batteries. Energy Environ Sci 2019, 12: 2433–2442.
Castle E, Csanádi T, Grasso S, et al. Processing and properties of high-entropy ultra-high temperature carbides. Sci Rep 2018, 8: 8609.
Gild J, Zhang YY, Harrington T, et al. High-entropy metal diborides: A new class of high-entropy materials and a new type of ultrahigh temperature ceramics. Sci Rep 2016, 6: 37946.
Tsai CW, Lai SW, Cheng KH, et al. Strong amorphization of high-entropy AlBCrSiTi nitride film. Thin Solid Films 2012, 520: 2613–2618.
Liu RH, Chen HY, Zhao KP, et al. Entropy as a gene-like performance indicator promoting thermoelectric materials. Adv Mater 2017, 29: 1702712.
Sarkar A, Djenadic R, Wang D, et al. Rare earth and transition metal based entropy stabilised perovskite type oxides. J Eur Ceram Soc 2018, 38: 2318–2327.
Qiu N, Chen H, Yang ZM, et al. A high entropy oxide (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2O) with superior lithium storage performance. J Alloys Compd 2019, 777: 767–774.
Tietz F, Fronia C. Polyanionic lattice modifications leading to high-entropy sodium ion conductors: Mathematical solution of accessible compositions. ChemPhysChem 2020, 21: 2096–2103.
Ma YJ, Ma Y, Dreyer SL, et al. High-entropy metal-organic frameworks for highly reversible sodium storage. Adv Mater 2021, 33: 2101342.
Usharani NJ, Shringi R, Sanghavi H, et al. Role of size, alio-/multi-valency and non-stoichiometry in the synthesis of phase-pure high entropy oxide (Co,Cu,Mg,Na,Ni,Zn)O. Dalton Trans 2020, 49: 7123–7132.
Yang LF, Chen C, Xiong S, et al. Multiprincipal component P2-Na0.6(Ti0.2Mn0.2Co0.2Ni0.2Ru0.2)O2 as a high-rate cathode for sodium-ion batteries. JACS Au 2021, 1: 98–107.
Wang JB, Cui YY, Wang QS, et al. Lithium containing layered high entropy oxide structures. Sci Rep 2020, 10: 18430.
Sarkar A, Wang QS, Schiele A, et al. High-entropy oxides: Fundamental aspects and electrochemical properties. Adv Mater 2019, 31: 1970189.
Rost CM, Sachet E, Borman T, et al. Entropy-stabilized oxides. Nat Commun 2015, 6: 8485.
Sarkar A, Breitung B, Hahn H. High entropy oxides: The role of entropy, enthalpy and synergy. Scripta Mater 2020, 187: 43–48.
Sarkar A, Breitung B, Hahn H. High entropy oxides: The role of entropy, enthalpy and synergy. Scripta Mater 2020, 187: 43–48.
Chen YW, Fu HY, Huang YY, et al. Opportunities for high-entropy materials in rechargeable batteries. ACS Mater Lett 2021, 3: 160–170.
Amiri A, Shahbazian-Yassar R. Recent progress of high-entropy materials for energy storage and conversion. J Mater Chem A 2021, 9: 782–823.
Yeh JW, Chen SK, Lin SJ, et al. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv Eng Mater 2004, 6: 299–303.
Ruffa AR. Thermal potential, mechanical instability, and melting entropy. Phys Rev B 1982, 25: 5895–5900.
Yeh JW. Alloy design strategies and future trends in high-entropy alloys. JOM 2013, 65: 1759–1771.
Bao S, Luo SH, Wang ZY, et al. Novel P2-type concentration-gradient Na0.67Ni0.167Co0.167Mn0.67O2 modified by Mn-rich surface as cathode material for sodium ion batteries. J Power Sources 2018, 396: 404–411.
Yuan DD, He W, Pei F, et al. Synthesis and electrochemical behaviors of layered Na0.67[Mn0.65Co0.2Ni0.15]O2 microflakes as a stable cathode material for sodium-ion batteries. J Mater Chem A 2013, 1: 3895.
Hou PY, Yin JM, Lu XH, et al. A stable layered P3/P2 and spinel intergrowth nanocomposite as a long-life and high-rate cathode for sodium-ion batteries. Nanoscale 2018, 10: 6671–6677.
Thorne JS, Dunlap RA, Obrovac MN. Structure and electrochemistry of NaxFexMn1−xO2 (1.0 ≤ x ≤ 0.5) for Na-ion battery positive electrodes. J Electrochem Soc 2012, 160: A361–A367.
Yu TY, Hwang JY, Aurbach D, et al. Microsphere Na0.65[Ni0.17Co0.11Mn0.72]O2 cathode material for high-performance sodium-ion batteries. ACS Appl Mater Interfaces 2017, 9: 44534–44541.
Li ZY, Gao R, Sun LM, et al. Designing an advanced P2-Na0.67Mn0.65Ni0.2Co0.15O2 layered cathode material for Na-ion batteries. J Mater Chem A 2015, 3: 16272–16278.
Zhou YN, Wang PF, Niu YB, et al. A P2/P3 composite layered cathode for high-performance Na-ion full batteries. Nano Energy 2019, 55: 143–150.
Ramasamy HV, N Didwal P, Sinha S, et al. Atomic layer deposition of Al2O3 on P2-Na0.5Mn0.5Co0.5O2 as interfacial layer for high power sodium-ion batteries. J Colloid Interface Sci 2020, 564: 467–477.
Zhou CJ, Yang LC, Zhou CG, et al. Co-substitution enhances the rate capability and stabilizes the cyclic performance of O3-type cathode NaNi0.45−xMn0.25Ti0.3CoxO2 for sodium-ion storage at high voltage. ACS Appl Mater Interfaces 2019, 11: 7906–7913.
Yan SX, Luo SH, Feng J, et al. Rational design of flower-like FeCo2S4/reduced graphene oxide films: Novel binder-free electrodes with ultra-high conductivity flexible substrate for high-performance all-solid-state pseudocapacitor. Chem Eng J 2020, 381: 122695.
Zhang QY, Zhang CL, Li B, et al. Preparation and characterization of W-doped Li4Ti5O12 anode material for enhancing the high rate performance. Electrochimica Acta 2013, 107: 139–146.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 51674068, 51874079, 51804035, and 11775226), the Natural Science Foundation of Hebei Province (No. E2018501091), the Hebei Province Key Research and Development Plan Project (No. 19211302D), the Fundamental Research Funds for the Central Universities (Nos. N172302001, N182306001, N182312007, N182304018, and N2023040), and the Research Project on the Distribution of Heavy Metals in Soil and Comprehensive Utilization Technology of Tailings in Typical Iron Tailing Reservoir Areas of Hebei Province (No. 802060671901).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yan, S., Luo, S., Yang, L. et al. Novel P2-type layered medium-entropy ceramics oxide as cathode material for sodium-ion batteries. J Adv Ceram 11, 158–171 (2022). https://doi.org/10.1007/s40145-021-0524-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40145-021-0524-8