Abstract
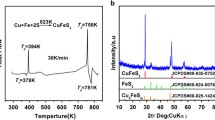
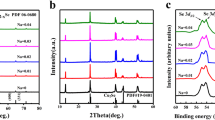
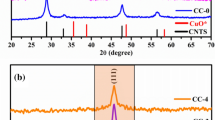
Chalcostibite (CuSbS2) is composed of earth-abundant elements and has a proper band gap (Eg = 1.05 eV) as a thermoelectric (TE) material. Herein, we report the TE properties in the CuSbS2based composites with a mole ratio of (1−x)CuSbS2−xCu18S (x = 0, 0.1, 0.2, 0.3), which were prepared by mechanical alloying (MA) combined with spark plasma sintering (SPS). X-ray diffraction (XRD) and back-scattered electron image (BSE) results indicate that a single phase of CuSbS2 is synthesized at x = 0 and the samples consist of CuSbS2, Cu3SbS4, and Cu12Sb4S13 at 0.1 ⩽ x ⩽ 0.3. The correlation between the phase structure, microstructure, and TE transport properties of the bulk samples is established. The electrical conductivity increases from 0.14 to 50.66 S·cm−1 at 723 K and at 0 ⩽ x ⩽ 0.03, while the Seebeck coefficient holds an appropriate value of 190.51 µV·K−1. The highest ZT value of 0.17 is obtained at 723 K and at x = 0.3 owing to the combination of a high PF 183 µW·m−1·K−2 and a low κ 0.8 W·m−1·K−1.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Huang SY, Xu XF. A regenerative concept for thermoelectric power generation. Appl Energy 2017, 185: 119–125.
Ge ZH, Zhao LD, Wu D, et al. Low-cost, abundant binary sulfides as promising thermoelectric materials. Mater Today 2016, 19: 227–239.
Gassoumi A, H.-E MMS, Alfaify S, et al. The investigation of crystal structure, elastic and optoelectronic properties of CuSbS2 and CuBiS2 compounds for photovoltaic applications. J Alloys Compd 2017, 725: 181–189.
Chen K. Synthesis and thermoelectric properties of Cu-Sb-S compounds. Ph.D. Thesis. London (UK): Queen Mary, University of London, 2016.
Du BL, Zhang RZ, Chen K, et al. The impact of lone-pair electrons on the lattice thermal conductivity of the thermoelectric compound CuSbS2. J Mater Chem A 2017, 5: 3249–3259.
Chen K, Du BL, Bonini N, et al. Theory-guided synthesis of an eco-friendly and low-cost copper based sulfide thermoelectric material. J Phys Chem C 2016, 120: 27135–27140.
Heo J, Laurita G, Muir S, et al. Enhanced thermoelectric performance of synthetic tetrahedrites. Chem Mater 2014, 26: 2047–2051.
Lu X, Morelli DT, Xia Y, et al. High performance thermoelectricity in earth-abundant compounds based on natural mineral tetrahedrites. Adv Energy Mater 2013, 3: 342–348.
Lu X, Morelli D. The effect of Te substitution for Sb on thermoelectric properties of tetrahedrite. J Electron Mater 2014, 43: 1983–1987.
Kumar Gudelli V, Kanchana V, Vaitheeswaran G, et al. Thermoelectric properties of chalcopyrite type CuGaTe2 and chalcostibite CuSbS2. J Appl Phys 2013, 114: 223707.
Rodríguez-Lazcano Y, Nair MTS, Nair PK. CuSbS2 thin film formed through annealing chemically deposited Sb2S3-CuS thin films. J Cryst Growth 2001, 223: 399–406.
Rodríguez-Lazcano Y, Nair MTS, Nair PK. Photovoltaic p-i-N structure of Sb2S3 and CuSbS2 absorber films obtained via chemical bath deposition. J Electrochem Soc 2005, 152: G635.
Rabhi A, Kanzari M. Structural, optical and electrical properties of CuSbS2 these amorphous films: effect of the thickness variation. Chalcogenide Lett 2011, 8: 383–390.
Liang DD, Ge ZH, Li HZ, et al. Enhanced thermoelectric property in superionic conductor Bi-doped Cu1.8S. J Alloys Compd 2017, 708: 169–174.
Ge ZH, Zhang BP, Chen YX, et al. Synthesis and transport property of Cu1.8S as a promising thermoelectric compound. Chem Commun 2011, 47: 12697–12699.
Yao Y, Zhang BP, Pei J, et al. Improved thermoelectric transport properties of Cu1.8S with NH4Cl-derived mesoscale-pores and point-defects. Ceram Int 2016, 42: 17518–17523.
Li JF, Pan Y, Wu CF, et al. Processing of advanced thermoelectric materials. Sci China Technol Sci 2017, 60: 1347–1364.
Saal JE, Kirklin S, Aykol M, et al. Materials design and discovery with high-throughput density functional theory: The open quantum materials database (OQMD). JOM 2013, 65: 1501–1509.
Kirklin S, Saal JE, Meredig B, et al. The open quantum materials database (OQMD): Assessing the accuracy of DFT formation energies. npj Comput Mater 2015, 1: 15010.
Zou L, Zhang BP, Ge ZH, et al. Size effect of SiO2 on enhancing thermoelectric properties of Cu1.8S. Phys Status Solidi A 2013, 210: 2550–2555.
Zheng LJ, Zhang BP, Li HZ, et al. CuxS superionic compounds: Electronic structure and thermoelectric performance enhancement. J Alloys Compd 2017, 722: 17–24.
Ge ZH, Zhang BP, Yu YQ, et al. Fabrication and properties of Bi2−xAg3xS3 thermoelectric polycrystals. J Alloys Compd 2012, 514: 205–209.
Goldsmid HJ. Introduction to Thermoelectricity. Berlin (Germany): Springer-Verlag Berlin Heidelberg, 2010.
Pei J, Zhang BP, Li JF, et al. Maximizing thermoelectric performance of AgPbmSbTem +2 by optimizing spark plasma sintering temperature. J Alloys Compd 2017, 728: 694–700.
Acknowledgements
This work was supported by National Key R&D Program of China (Grant No. 2018YFB0703600) and the National Natural Science Foundation of China (Grant No. 11474176).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tang, C., Liang, D., Li, H. et al. Preparation and thermoelectric properties of Cu1.8S/CuSbS2 composites. J Adv Ceram 8, 209–217 (2019). https://doi.org/10.1007/s40145-018-0306-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40145-018-0306-0