Abstract
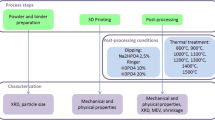
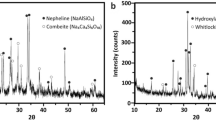
This paper characterizes in an in vitro setting the release of calcium (Ca) and phosphate (PO4) of 3D printed bioactive ceramic scaffold prepared from extrudable paste containing hydroxyapatite and β-tricalcium phosphate (β-TCP). Hydroxyapatite and β-TCP were calcined at 800 °C for 11 h, fabricated into four experimental groups (100% HA, 100% β-TCP, 15%/85% HA/β-TCP, and 15%/85% HA/β-TCP (design)), sintered to 1100 °C for 4 h. Calcium and phosphorus concentrations were evaluated using ICP spectroscopy, and the release of Ca and PO4 ions during dissolution of the CaP-based scaffolds was measured by submerging in 0.05 mol/L Tris(hydroxymethyl)aminomethane-HCl and maintaining a temperature of 37 °C. The Ca and PO4 concentrations of the solutions were measured with the utilization of a calcium assay kit and a phosphate assay kit and read in a UV–visible spectrophotometer. The 100% HA scaffold group showed the greatest concentration of Ca ions (~1.9 mg/dL), but ultimately released at a lower amount as time increased; the 100% HA scaffold also showed the lowest total amount of calcium ions released over the course of evaluation. The results for the 100% β-TCP were on the opposite of the HA with the highest amount of calcium ion release over the study. While the PO4 ion release showed a similar trend as those observed with Ca ions with an apparent difference in the 100% HA scaffold group. There was nearly 0 mg/dL of the phosphate ions released in the first 24 h, in comparison to the amount of Ca ions released during the same time frame. Since various formulations can lead to different properties of these bioactive ceramic scaffolds, it is important to understand how the tailoring of this important biphasic material can impact the long-term outcome of an ever-important in vivo clinical trial in the future.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
LeGeros RZ, Parsons JR, Daculsi G, et al. Significance of the porosity and physical chemistry of calcium phosphate ceramics biodegradation–bioresorption. Ann NY Acad Sci 1988, 523: 268–271.
Daculsi G, LeGeros RZ, Nery E, et al. Transformation of biphasic calcium phosphate ceramics in vivo: Ultrastructural and physicochemical characterization. J Biomed Mater Res 1989, 23: 883–894.
Heughebaert M, LeGeros RZ, Gineste M, et al. Physicochemical characterization of deposits associated with HA ceramics implanted in nonosseous sites. J Biomed Mater Res 1988, 22: 257–268.
Ducheyne P, Radin S, King L. The effect of calcium phosphate ceramic composition and structure on in vitro behavior. I. Dissolution. J Biomed Mater Res 1993, 27: 25–34.
Duheyne P, Beight J, Cuckler J, et al. Effect of calcium phosphate coating characteristics on early post-operative bone tissue ingrowth. Biomaterials 1990, 11: 531–540.
Ducheyne P, Cuckler JM. Bioactive ceramic prosthetic coatings. Clin Orthop Relat R 1992, 76: 102–114.
Witek L, Smay J, Silva NRFA, et al. Sintering effects on the chemical and physical properties of bioactive ceramic rods biomaterials and biomimetics. New York University, 2011.
Radin SR, Ducheyne P. Plasma spraying induced changes of calcium phosphate ceramic characteristics and the effect on in vitro stability. J Mater Sci: Mater Med 1992, 3: 33–42.
De Groot K, Klein C, Wolke JGC, et al. Plasma-sprayed coatings of calcium phosphate. CRC Handbook of Bioactive Ceramics 1990, 2: 133–142.
Vasiliu CEC. Assembly of hydroxy apatite: β tricalcium phosphate: Calcium sulfate bone engineering scaffolds. Master Thesis. Oklahoma State University, 2008.
Szpalski C, Nguyen PD, Vasiliu CEC, et al. Bony engineering using time-release porous scaffolds to provide sustained growth factor delivery. J Craniofac Surg 2012, 23: 638–644.
Simon JL, Michna S, Lewis JA, et al. In vivo bone response to 3D periodic hydroxyapatite scaffolds assembled by direct ink writing. J Biomed Mater Res 2007, 83A: 747–758.
Mijares DQ. Synthetic bone mineral (SBM): Prevention of bone loss induced by estrogen deficiency in a rat model. New York: NYU College of Dentistry, 2009.
Taylor JC, Hinczak I. Rietveld made easy: A practical guide to the understanding of the method and successful phase quantifications. Sietronics Pty Limited, 2006. Available at http://www.ccp14.ac.uk/ccp/web-mirrors/commercial/siro quant/siroqnt/pdfs/rmeintro.pdf.
Rietveld HM. The Rietveld Method. Oxford University Press, 1993.
Nilen RWN, Richter PW. The thermal stability of hydroxyapatite in biphasic calcium phosphate ceramics. J Mater Sci: Mater Med 2008, 19: 1693–1702.
Daculsi G. Biphasic calcium phosphate concept applied to artificial bone, implant coating and injectable bone substitute. Biomaterials 1998, 19: 1473–1478.
Amera A, Abudalazez AMA, Ismail RA, et al. Synthesis and characterization of porous biphasic calcium phosphate scaffold from different porogens for possible bone tissue engineering applications. Sci Sinter 2011, 43: 183–192.
Moore WR, Graves SE, Bain GI. Synthetic bone graft substitutes. ANZ J Surg 2001, 71: 354–361.
Miranda P, Saiz E, Gryn K, et al. Sintering and robocasting of β-tricalcium phosphate scaffolds for orthopaedic applications. Acta Biomater 2006, 2: 457–466.
Gaasbeek A, Meinders AE. Hypophosphatemia: An update on its etiology and treatment. Am J Med 2005, 118: 1094–1101.
Razzaque MS. The FGF23–Klotho axis: Endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol 2009, 5: 611–619.
Iotti S, Lodi R, Gottardi G, et al. Inorganic phosphate is transported into mitochondria in the absence of ATP biosynthesis: An in vivo 31P NMR study in the human skeletal muscle. Biochem Bioph Res Co 1996, 225: 191–194.
Hutson SM, Williams GD, Berkich DA, et al. A phosphorus-31 NMR study of mitochondrial inorganic phosphate visibility: Effects of calcium(2+) and manganese(2+) and the pH gradient. Biochemistry 1992, 31: 1322–1330.
Razzaque M. Phosphate toxicity: New insights into an old problem. Clin Sci 2011, 120: 91–97.
Fukagawa M, Hamada Y, Nakanishi S, et al. The kidney and bone metabolism: Nephrologists’ point of view. J Bone Miner Metab 2006, 24: 434–438.
Razzaque MS. Therapeutic potential of klotho-FGF23 fusion polypeptides: WO2009095372. Expert Opin Ther Pat 2010, 20: 981–985.
Chen J, Xu L, Chen S, et al. Transcriptional regulation of platelet-derived growth factor-B chain by thrombin in endothelial cells: Involvement of Egr-1 and CREB-binding protein. Mol Cell Biochem 2012, 366: 81–87.
Sweet L, Kang Y, Czisch C, et al. Geometrical versus random β-TCP scaffolds: Exploring the effects on Sschwann cell growth and behavior. PLoS ONE 2015, 10: e0139820.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at Springerlink.com
Rights and permissions
Open Access The articles published in this journal are distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Witek, L., Shi, Y. & Smay, J. Controlling calcium and phosphate ion release of 3D printed bioactive ceramic scaffolds: An in vitro study. J Adv Ceram 6, 157–164 (2017). https://doi.org/10.1007/s40145-017-0228-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40145-017-0228-2