Abstract
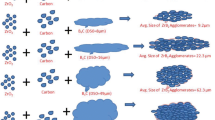
ZrB2 and ZrB2–SiC powders have been produced by reducing ZrO2 and ZrSiO4 with B4C without using any furnace. Magnesium was added to the mixtures of (ZrO2+B4C) and (ZrSiO4+B4C). The reaction has been assisted by a floral thermite packed around the compacts. By introducing elemental Si into (ZrO2+B4C) mixture, composite powders of ZrB2–SiC formed. After leaching out MgO with suitable HCl water solution, the product was analysed by X-ray diffraction (XRD) and scanning electron microscopy (SEM). The effect of Si, B4C, and Mg on the extent of formation of ZrB2, ZrB2–SiC, and other phases has been studied. Formation of nano-sized ZrB2 and ZrB2–SiC composite powders was identified. The adaptability of the process for bulk production was examined.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Opeka MM, Talmy IG, Zaykoski JA. Oxidation-based materials selection for 2000 °C hypersonic aerosurfaces: Theoretical considerations and historical experience. J Mater Sci 2004, 39: 5887–5904.
Jackson TA, Ekulund DR, Fink AJ. High speed propulsion: Performance and advantage of advanced materials. J Mater Sci 2004, 39: 5905–5913.
Fahrenholtz WG, Hilmas GE, Talmy IG, et al. Refractory diborides of zirconium and hafnium. J Am Ceram Soc 2007, 90: 1347–1364.
Peshev P, Bliznakov G. On the borothermic preparation of titanium, zirconium and hafnium borides. J Less-Common Met 1968, 14: 23–32.
Zhao H, He Y, Jin Z. Preparation of zirconium boride powder. J Am Ceram Soc 1995, 78: 2534–2536.
Guo W-M, Zhang G-J. Reaction processes and characterization of ZrB2 powder prepared by boro carbothermal reduction of ZrO2 in vacuum. J Am Ceram Soc 2009, 92: 264–267.
Chen L, Gu Y, Yang Z, et al. Preparation and some properties of nano crystalline ZrB2 powders. Scripta Mater 2004, 50: 959–961.
Radev DD, Marinov M. Properties of titanium and zirconium diborides obtained by self-propagated high-temperature synthesis. J Alloys Compd 1996, 244: 48–51.
Thompson M, Fahrenholtz WG, Hilmas G. Effect of starting particle size and oxygen content on densification of ZrB2. J Am Ceram Soc 2011, 94: 429–435.
Zhu S, Fahrenholtz WG, Hilmas GE, et al. Pressureless sintering of carbon-coated zirconium diboride powders. Mat Sci Eng A 2007, 459: 167–171.
Zou J, Zhang G-J, Zhang H, et al. Improving high temperature properties of hot pressed ZrB2–20 vol% SiC ceramic using high purity powders. Ceram Int 2013, 39: 871–876.
Qiu H-Y, Guo W-M, Zou J, et al. ZrB2 powders prepared by boro/carbothermal reduction ZrO2: The effects of carbon source and reaction atmosphere. Powder Technol 2012, 217: 462–466.
Krishnarao RV, Alam MdZ, Das DK, et al. Synthesis of ZrB2–SiC composite powder in air furnace. Ceram Int 2014, 40: 15647–15653.
Khanra AK, Pathak LC, Godkhindi MM. Double SHS of ZrB2 powder. J Mater Process Tech 2008, 202: 386–390.
Çamurlu HE, Maglia F. Preparation of nano-size ZrB2 by self-propagating high-temperature synthesis. J Eur Ceram Soc 2009, 29: 1501–1506.
Tsuchida T, Yamamoto S. Mechanical activation assisted self-propagating high-temperature synthesis of ZrC and ZrB2 in air from Zr/B/C powder mixtures. J Eur Ceram Soc 2004, 24: 45–51.
Akkas B, Alkan M, Derin B, et al. Production of zirconium diboride powder by self propagating high temperature synthesis. Adv Sci Tech 2010, 63: 251–256.
Krishnarao RV. Preparation of ZrB2 and ZrB2–SiC powders in a single step reduction of zircon (ZrSiO4) with B4C. Ceram Int 2017, 43: 1205–1209.
Ryu HY, Nersisyan NH, Lee JH. Preparation of zirconium-based ceramic and composite fine-grained powders. Int J Refract Met H 2012, 30: 133–138.
Jalaly M, Bafghi MSh, Tamizifar M, et al. Mechanosynthesis of nanocrystalline ZrB2-based powders by mechanically induced self-sustaining reaction method. Adv Appl Ceram 2013, 112: 383–388.
Deng X, Du S, Zhang H, et al. Preparation and characterization of ZrB2–SiC composite powders from zircon via microwave assisted boro/crbothermal reduction. Ceram Int 2015, 41: 14419–14426.
Oh H-C, Lee S-H, Choi S-C. Two-step reduction process and spark plasma sintering for the synthesis of ultra fine SiC and ZrB2 powder mixtures. Int J Refract Met H 2014, 42: 132–135.
Barin I. Thermochemical Data of Pure Substances. Wiley-VCH, 1997.
Merzhanov AG. Self-propagating high temperature synthesis: Twenty years of research and findings. In: Combustion and Plasma Synthesis of High Temperature Materials. Munir Z, Holt IB, Eds. New York: VCH, 1990: 1–53.
Mukasyan AS. Combustion synthesis of silicon carbide. In Properties and Applications of Silicon Carbide. Gerhardt R, Ed. Vienna, Austria: INTECH, 2011: 389–409.
Pampuch R, Stobierski L, Liz J. Synthesis of sinterable β-SiC powders by solid combustion method. J Am Ceram Soc 1989, 72: 1434–1435.
Barton L, Nicholls D. The hydrogenation of boron monoxide to diborane and the reactions of boron and boron carbide with titanium and zirconium dioxides. J Inorg Nucl Chem 1996, 28: 1367–1372.
Ran S, van der Biest O, Vleugel J. ZrB2 powders synthesis by borothermal reduction. J Am Ceram Soc 2010, 93: 1586–1590.
Guo W-M, Tan D-W, Zhang Z-L, et al. Synthesis of fine ZrB2 powders by new borothermal reduction of coarse ZrO2 powders. Ceram Int 2016, 42: 15087–15090.
Acknowledgements
Authors acknowledge the financial support from the Defence Research and Development Organization, Ministry of Defence, Government of India, New Delhi, in order to carry out the present study under project 295. They are grateful to the Director, Defence Metallurgical Research Laboratory, Hyderabad, for his constant encouragement. The authors acknowledge the support of ACG, XRD, and SEM groups of Defence Metallurgical Research Laboratory.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at Springerlink.com
Rights and permissions
Open Access The articles published in this journal are distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Krishnarao, R.V., Sankarasubramanian, R. Thermite assisted synthesis of ZrB2 and ZrB2–SiC through B4C reduction of ZrO2 and ZrSiO4 in air. J Adv Ceram 6, 139–148 (2017). https://doi.org/10.1007/s40145-017-0226-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40145-017-0226-4