Abstract
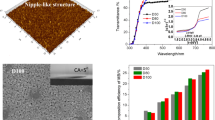
A hierarchical structural surface of TiO2 film with reversibly light-switchable wettability between superhydrophobicity and superhydrophilicity on metal substrate was fabricated through simply dip-coating method from TiO2 precursor solution containing TiO2 nanoparticles with the average diameter 25 nm (P25), followed by heat-treatment and modification with fluoroalkylsilane (FAS) molecules. The morphology, phase and crystallographic structure, and chemical composite of the as-prepared TiO2 film were characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS). The wettability of TiO2 film was characterized by a drop shape analyzer. The water contact angle of superhydrophobic TiO2 film was up to 165.6°. Under UV irradiation, the water contact angle decreased and the superhydrophobic TiO2 film became superhydrophilic because of hydroxyl groups absorption on the TiO2 surface. Meanwhile, the surface morphology of TiO2 film, which resulted from the TiO2 nanoparticles added in TiO2 precursor solution, had a significant effect on the wettability conversion of TiO2 film and enhanced the switch from hydrophobicity to hydrophilicity. The wettability could be reversibly switched between superhydrophobicity and superhydrophilicity via alternation of UV exposure and dark storage.
Article PDF
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Change history
02 March 2017
An erratum has been published.
References
Wang J-M, Wang K, Zheng Y-M, et al. Effects of chemical composition and nano-structrues on the wetting behaviour of lotus leaves. Chemical Journal of Chinese Universities 2010, 31: 1596–1599.
Callies M, Quéré D. On water repellency. Soft Matter 2005, 1: 55–61.
Sun T, Feng L, Gao X, et al. Bioinspired surfaces with special wettability. Acc Chem Res 2005, 38: 644–652.
Nosonovsky M, Bhushan B. Biologically inspired surfaces: Broadening the scope of roughness. Adv Funct Mater 2008, 18: 843–855.
Tuteja A, Choi W, Ma M, et al. Designing superoleophobic surfaces. Science 2007, 318: 1618–1622.
Zhang X, Shi F, Niu J, et al. Superhydrophobic surfaces: From structural control to functional application. J Mater Chem 2008, 18: 621–633.
Huang X, Zacharia NS. Functional polyelectrolyte multilayer assemblies for surfaces with controlled wetting behaviour. J Appl Polym Sci 2015, 132: 42767.
Liu Y, Wang X, Fei B, et al. Bioinspired, stimuli-responsive, multifunctional superhydrophobic surface with directional wetting, adhesion, and transport of water. Adv Funct Mater 2015, 25: 5047–5056.
Li J, Sun Q, Han S, et al. Reversibly light-switchable wettability between superhydrophobicityand superhydrophilicity of hybrid ZnO/bamboo surfaces via alternation of UV irradiation and dark storage. Prog Org Coat 2015, 87: 155–160.
Ju J, Xiao K, Yao X, et al. Bioinspired conical copper wire with gradient wettability for continuous and efficient fog collection. Adv Mater 2013, 25: 5937–5942.
Wang J, Geng G. Simple and eco-friendly fabrication of superhydrophobic textile for oil/water separation. Environ Technol 2016, 37: 1591–1596.
Liu X. Zinc oxide nano- and microfabrication from coordination-polymer templates. Angew Chem Int Edit 2009, 48: 3018–3021.
Song W, Veiga DD, Custódio CA, et al. Bioinspired degradable substrates with extreme wettability properties. Adv Mater 2009, 21: 1830–1834.
Yin J, Cao H. Synthesis and photocatalytic activity of single-crystalline hollow rh-In2O3 nanocrystals. Inorg Chem 2012, 51: 6529–6536.
Dalrymple OK, Stefanakos E, Trotz MA, et al. A review of the mechanisms and modeling of photocatalytic disinfection. Appl Catal B: Environ 2010, 98: 27–38.
Zhu X, Zhang Z, Men X, et al. Fabrication of an intelligent superhydrophobic surface based on ZnO nanorod arrays with switchable adhesion property. Appl Surf Sci 2010, 256: 7619–7622.
Xin B, Hao J. Reversibly switchable wettability. Chem Soc Rev 2010, 39: 769–782.
Liu K, Cao M, Fujishima A, et al. Bio-inspired titanium dioxide materials with special wettability and their applications. Chem Rev 2014, 114: 10044–10094.
Yadav K, Mehta BR, lakshmi KV, et al. Tuning the wettability of indium oxide nanowires: Effect of oxygen related defects. J Phys Chem C 2015, 119: 16026–16032.
Hoshian S, Jokinen V, Hjort K, et al. Amplified and localized photoswitching of TiO2 by micro- and nanostructuring. ACS Appl Mater Interfaces 2015, 7: 15593–15599.
Liu Y, Wang X, Fei B, et al. Bioinspired, stimuli-responsive, multifunctional superhydrophobic surface with directional wetting, adhesion, and transport of water. Adv Funct Mater 2015, 25: 5047–5056.
Hu Y-W, He H-R, Ma Y-M, et al. Fabrication and wettability conversion of ZnO/Ag composite films. Chemical Journal of Chinese Universities 2013, 34: 295–298.
Zhang R, Wu H, Lin D, et al. Photocatalytic and magnetic properties of the Fe–TiO2/SnO2 nanofiber via electrospinning. J Am Ceram Soc 2010, 93: 605–608.
Mishra A, Fischer MKR, Bäuerle P. Metal-free organic dyes for dye-sensitized solar cells: From structure: Property relationships to design rules. Angew Chem Int Edit 2009, 48: 2474–2499.
Zhang R, Wu H, Lin D, et al. Preparation of necklace-structured TiO2/SnO2 hybrid nanofibers and their photocatalytic activity. J Am Ceram Soc 2009, 92: 2463–2466.
Eshaghi A, Eshaghi A. Preparation and hydrophilicity of TiO2 sol–gel derived nanocomposite films modified with copper loaded TiO2 nanoparticles. Mater Res Bull 2011, 46: 2342–2345.
Fan X, Li X, Tian D, et al. Optoelectrowettability conversion on superhydrophobic CdS QDs sensitized TiO2 nanotubes. J Colloid Interface Sci 2012, 366: 1–7.
Hu Y, Huang S, Liu S, et al. A corrosion-resistance superhydrophobic TiO2 film. Appl Surf Sci 2012, 258: 7460–7464.
Feng L, Li SH, Li Y, et al. Super-hydrophobic surfaces: From natural to artificial. Adv Mater 2002, 14: 1857–1860.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 21201115), State Key Laboratory of New Ceramics and Fine Processing, Tsinghua University (No. KF201614), and Technology Foundation for Selected Overseas Chinese Scholar of Shaanxi Province of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at Springerlink.com
Rights and permissions
Open Access The articles published in this journal are distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kong, X., Hu, Y., Wang, X. et al. Effect of surface morphology on wettability conversion. J Adv Ceram 5, 284–290 (2016). https://doi.org/10.1007/s40145-016-0201-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40145-016-0201-5