Abstract
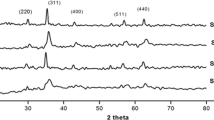
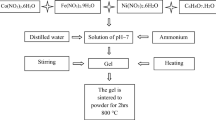
A detailed investigation on the effect of preparation method on the structural, magnetic, and acidic properties of cobalt ferrite nanoparticles prepared by sol–gel and co-precipitation is presented. Citric acid and ethylene glycol were used as gelling agents, while sodium hydroxide and aqueous ammonia were used as precipitating agents. The resulting ferrites were calcined at 450°C and 750°C. Sharper X-ray diffraction (XRD) peaks were observed for the samples calcined at 750°C, indicating greater crystallinity of the samples calcined at higher temperature. Average crystallite sizes fell in the ranges of 7.1–21.1 nm and 30.4–42.1 nm for the samples calcined at 450°C and 750°C, respectively. The infrared spectra revealed two main absorption bands, the high frequency band ν1 around 600 cm-1 and the low frequency band ν2 around 400 cm-1 arising from stretching vibrations of the oxygen bond with the metal in the tetrahedral (A) and octahedral (B) sites in the spinel lattice. Agglomeration of particles was observed in the scanning electron microscopy (SEM) images. Magnetic parameters of CoFe2O4 nanoparticles greatly depended on calcination temperature and preparation techniques. Ammonia temperature programmed desorption (TPD) measurements indicated that weak acid sites predominate medium strength sites, while the number of strong acid sites is the least. Cumulative acidity decreased for the samples calcined at higher temperature. The results underline the effect of preparation conditions on the morphology, crystallite size, and magnetic properties of nano ferrites.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Maaz K, Mumtaz A, Hasanain SK. Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J Magn Magn Mater 2007, 308: 289–295.
Zhao D, Wu X, Guan H, et al. Study on supercritical hydrothermal synthesis of CoFe2O4 nanoparticles. The Journal of Supercritical Fluids 2007, 42: 226–233.
Giri AK, Kirkpatric EM, Moongkhmklang P, et al. Photomagnetism and structure in cobalt ferrite nanoparticles. Appl Phys Lett 2002, 80: 2341.
Bueno AR, Gregori ML, Nóbrega MCS. Effect of Mn substitution on the microstructure and magnetic properties of Ni0.50-x Zn0.50-x Mn2x Fe2O4 ferrite prepared by the citrate–nitrate precursor method. Mater Chem Phys 2007, 105: 229–233.
[5] Costa ACFM, Silva VJ, Cornejo DR, et al. Magnetic and structural properties of NiFe2O4 ferrite nanopowder doped with Zn2+. J Magn Magn Mater 2008, 320: e370–e372.
Kurian M, Nair DS, Rahnamol AM. Influence of the synthesis conditions on the catalytic efficiency of NiFe2O4 and ZnFe2O4 nanoparticles towards the wet peroxide oxidation of 4-chlorophenol. Reaction Kinetics, Mechanisms and Catalysis 2014, 111: 591–604.
Kundu TK, Mishra S. Nanocrystalline spinel ferrites by solid state reaction route. Bull Mater Sci 2008, 31: 507–510.
Ma R, Wang Y, Zhang C, et al. Microwave absorbing properties of magnesium-substituted MnZn ferrites prepared by citrate-EDTA complexing method. J Mater Sci Technol 2008, 24: 419.
Kurian M, Nair DS. Synthesis and characterization of nickel zinc ferrite. AIP Conf Proc 2011, 1391: 594.
Sajjia M, Oubaha M, Hasanuzzaman M, et al. Developments of cobalt ferrite nanoparticles prepared by the sol–gel process. Ceram Int 2014, 40: 1147–1154.
Feng H, Chen B, Zhang D, et al. Preparation and characterization of the cobalt ferrite nano-particles by reverse coprecipitation. J Magn Magn Mater 2014, 356: 68–72.
Kurian M, Nair DS. On the efficiency of cobalt zinc ferrite nanoparticles for catalytic wet peroxide oxidation of 4-chlorophenol. Journal of Environmental Chemical Engineering 2014, 2: 63–69.
Sorescu M, Diamandescu L, Peelamedu R, et al. Structural and magnetic properties of NiZn ferrites prepared by microwave sintering. J Magn Magn Mater 2004, 279: 195–201.
Jovanovic S, Spreitzer M, Otonicar M, et al. pH control of magnetic properties in precipitation-hydrothermal-derived CoFe2O4. J Alloys Compd 2014, 589: 271–277.
Liu M, Obi O, Lou J, et al. Strong magnetoelectric coupling in ferrite / ferroelectric multiferroic heterostructures derived by low temperature spin-spray deposition. J Phys D: Appl Phys 2009, 42: 045007.
Abbas M, Rao BP, Islam MN, et al. Size-controlled high magnetization CoFe2O4 nanospheres and nanocubes using rapid one-pot sonochemical technique. Ceram Int 2014, 40: 3269–3276.
Kurian M, Nair DS. Effect of preparation conditions on nickel zinc ferrite nanoparticles: A comparison between sol–gel auto combustion and co-precipitation methods. Journal of Saudi Chemical Society 2013, DOI: 10.1016/j.jscs.2013.03.003.
Rezlescu N, Rezlescu E, Popa PD, et al. Comparative study between catalyst properties of simple spinel ferrite powders prepared by self-combustion route. Romanian Reports in Physics 2013, 65: 1348–1356.
Vaidyanathan G, Sendhilnathan S, Arulmurugan R. Structural and magnetic properties of Co1-x Zn x Fe2O4 nanoparticles by co-precipitation method. J Magn Magn Mater 2007, 313: 293–299.
Thomas M, George KC. Infrared and magnetic study of nanophase zinc ferrite. Indian J Pure Ap Phy 2009, 47: 81–86.
Sathishkumar G, Venkataraju C, Sivakumar K. Effect of nickel on the structural and magnetic properties of nano structured CoZnFe2O4. J Mater Sci: Mater El 2011, 22: 1715–1724.
Gopalan EV, Joy PA, Al-Omari IA, et al. On the structural, magnetic and electrical properties of sol–gel derived nanosized cobalt ferrite. J Alloys Compd 2009, 485: 711–717.
Khot SS, Shinde NS, Ladgaonkar BP, et al. Magnetic and structural properties of magnesium zinc ferrites synthesized at different temperature. Adv Appl Sci Res 2011, 2: 460–471.
Chakraverty S, Bandyopadhyay M. Coercivity of magnetic nanoparticles: A stochastic model. J Phys: Condens Matter 2007, 19: 216201.
Ramankutty CG, Sugunan S, Thomas B. Study of cyclohexanol decomposition reaction over the ferrospinels A1-x Cu x Fe2O4 (A = Ni or Co and x = 0, 0.3, 0.5, 0.7 and 1) prepared by ‘soft’ chemical methods. J Mol Catal A: Chem 2002, 187: 105–117.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at Springerlink.com
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kurian, M., Thankachan, S., Nair, D.S. et al. Structural, magnetic, and acidic properties of cobalt ferrite nanoparticles synthesised by wet chemical methods. J Adv Ceram 4, 199–205 (2015). https://doi.org/10.1007/s40145-015-0149-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40145-015-0149-x