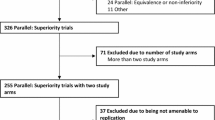
Abstract
Purpose of Review
Randomized controlled trials (RCT) are recognized as the most robust design to study the relationship between exposure and outcomes. The conventional RCT design is commonly used in pharmacological trials. Some surgical interventions are not be well suited to a conventional RCT design and may be associated with methodological challenges. Approaches have been proposed in non-pharmacological trials to overcome some of these challenges and minimize the risk of bias.
Recent Findings
Imbalance in prognostic factors between intervention groups, lack of allocation concealment, unblinding, non-intention-to-treat analysis, and losses to follow-ups can all threaten the validity of RCT results to various degrees. Procedure-based trials raise also specific challenges since physician expertise and training can affect the intervention, exposing to potential differential-expertise bias. Lack of statistical power can also affect the confidence in a trial’s result. Small sample sizes also usually mean small number of events for comparison between interventions, resulting in less statistically robust findings.
Summary
Minimizing risk of bias and achieving adequate statistical power are crucial to producing high quality and meaningful results. Non-pharmacological trials pose certain methodological challenges, and several approaches have been proposed to address the risk of bias. Large sample sizes are also usually required to achieve sufficient statistical power to provide answers to meaningful clinical questions. However, small perioperative trials remain frequent and result interpretation based solely on P values might not always appropriately inform on the confidence in a trial’s results. The Fragility Index can be used to further inform on the confidence of statistically significant result.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as • Of importance •• Of major importance
Devereaux PJ, Yusuf S. The evolution of the randomized controlled trial and its role in evidence-based decision making. J Intern Med. 2003;254(2):105–13.
Sackett DL. Bias in analytic research. J Chronic Dis. 1979;32(1–2):51–63.
Group GW. Grading quality of evidence and strength of recommendations. BMJ Br Med J. 2004;328(7454):1490.
Farrokhyar F, Karanicolas PJ, Thoma A, Simunovic M, Bhandari M, Devereaux PJ, et al. Randomized controlled trials of surgical interventions. Ann Surg. 2010;251(3):409–16.
Schulz KF, Grimes DA. Generation of allocation sequences in randomised trials: chance, not choice. Lancet. 2002;359(9305):515–9.
Lachin JM. Properties of simple randomization in clinical trials. Control Clin Trials. 1988;9(4):312–26.
Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. Lancet. 2002;359(9306):614–8.
Schulz KF, Chalmers I, Grimes DA, Altman DG. Assessing the quality of randomization from reports of controlled trials published in obstetrics and gynecology journals. JAMA. 1994;272(2):125–8.
Altman DG, Dore CJ. Randomisation and baseline comparisons in clinical trials. Lancet. 1990;335(8682):149–53.
Meinert CL, Tonascia S. Clinical trials: design, conduct, and analysis. Oxford: Oxford University Press; 1986.
Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D, Sculpher MJ. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 2010;362(20):1863–71.
Montenij L, de Waal E, Frank M, van Beest P, de Wit A, Kruitwagen C, et al. Influence of early goal-directed therapy using arterial waveform analysis on major complications after high-risk abdominal surgery: study protocol for a multicenter randomized controlled superiority trial. Trials. 2014;15:360.
Buse GL, et al. Accelerated care versus standard care among patients with hip fracture: the HIP ATTACK pilot trial. Cmaj. 2014;186(1):52–60.
Herbison P, Hay-Smith J, Gillespie WJ. Different methods of allocation to groups in randomized trials are associated with different levels of bias. A meta-epidemiological study. J Clin Epidemiol. 2011;64(10):1070–5.
•• Evaniew N, Carrasco-Labra A, Devereaux PJ, Tikkinen KA, Fei Y, Bhandari M, et al. How to use a randomized clinical trial addressing a surgical procedure: users’ guide to the medical literature. JAMA Surg. 2016. - This publication of the Users Guide to the Medical Literature RCTs provides comprehensible review of use and misuse of RCTs in the surgical setting.
Greenfield ML, Mhyre JM, Mashour GA, Blum JM, Yen EC, Rosenberg AL. Improvement in the quality of randomized controlled trials among general anesthesiology journals 2000 to 2006: a 6-year follow-up. Anesth Analg. 2009;108(6):1916–21.
Voineskos SH, Coroneos CJ, Ziolkowski NI, Kaur MN, Banfield L, Meade MO, et al. A systematic review of surgical randomized controlled trials: Part I. Risk of bias and outcomes: common pitfalls plastic surgeons can overcome. Plast Reconstr Surg. 2016;137(2):696–706.
Devereaux PJ, Choi PT, El-Dika S, Bhandari M, Montori VM, Schunemann HJ, et al. An observational study found that authors of randomized controlled trials frequently use concealment of randomization and blinding, despite the failure to report these methods. J Clin Epidemiol. 2004;57(12):1232–6.
Moseley JB, O’Malley K, Petersen NJ, Menke TJ, Brody BA, Kuykendall DH, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347(2):81–8.
Wei JT, Nygaard I, Richter HE, Nager CW, Barber MD, Kenton K, et al. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366(25):2358–67.
Koutsourelakis I, Georgoulopoulos G, Perraki E, Vagiakis E, Roussos C, Zakynthinos SG. Randomised trial of nasal surgery for fixed nasal obstruction in obstructive sleep apnoea. Eur Respir J. 2008;31(1):110–7.
Horng S, Miller FG. Ethical framework for the use of sham procedures in clinical trials. Crit Care Med. 2003;31(3 Suppl):S126–30.
Wolf BR, Buckwalter JA. Randomized surgical trials and “sham” surgery: relevance to modern orthopaedics and minimally invasive surgery. Iowa Orthop J. 2006;26:107–11.
Dowrick AS, Bhandari M. Ethical issues in the design of randomized trials: to sham or not to sham. J Bone Joint Surg Am. 2012;94(Suppl 1):7–10.
Sackett DL. Clinician-trialist rounds: 5. Cointervention bias–how to diagnose it in their trial and prevent it in yours. Clin Trials. 2011;8(4):440–2.
Hrobjartsson A, Emanuelsson F, Skou Thomsen AS, Hilden J, Brorson S. Bias due to lack of patient blinding in clinical trials. A systematic review of trials randomizing patients to blind and nonblind sub-studies. Int J Epidemiol. 2014;43(4):1272–83.
Hrobjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non-blinded outcome assessors. BMJ. 2012;344:e1119.
Hrobjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. CMAJ. 2013;185(4):E201–11.
Poolman RW, Struijs PA, Krips R, Sierevelt IN, Marti RK, Farrokhyar F, et al. Reporting of outcomes in orthopaedic randomized trials: does blinding of outcome assessors matter? J Bone Joint Surg Am. 2007;89(3):550–8.
Majeed AW, Troy G, Nicholl JP, Smythe A, Reed MW, Stoddard CJ, et al. Randomised, prospective, single-blind comparison of laparoscopic versus small-incision cholecystectomy. Lancet. 1996;347(9007):989–94.
Devereaux PJ, Mrkobrada M, Sessler DI, Leslie K, Alonso-Coello P, Kurz A, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494–503.
Vannabouathong C, Saccone M, Sprague S, Schemitsch EH, Bhandari M. Adjudicating outcomes: fundamentals. J Bone Joint Surg Am. 2012;94(Suppl 1):70–4.
Torgerson DJ. Contamination in trials: is cluster randomisation the answer? BMJ: Br Med J. 2001;322(7282):355–7.
Cook JA, McCulloch P, Blazeby JM, Beard DJ, Marinac-Dabic D, Sedrakyan A. IDEAL framework for surgical innovation 3: randomised controlled trials in the assessment stage and evaluations in the long term study stage. BMJ. 2013;346:f2820.
Devereaux PJ, Bhandari M, Clarke M, Montori VM, Cook DJ, Yusuf S, et al. Need for expertise based randomised controlled trials. BMJ. 2005;330(7482):88.
• Cook JA, Elders A, Boachie C, Bassinga T, Fraser C, Altman DG, et al. A systematic review of the use of an expertise-based randomised controlled trial design. Trials. 2015;16:241. - A systematic review that informs on the current use of expertise-based design in RCTs. Expertise-based design has gained popularity in the last decade as a novel approach to conduct RCT, especially in non-pharmacological and surgical trials.
Walter SD, Ismaila AS, Devereaux PJ. Statistical issues in the design and analysis of expertise-based randomized clinical trials. Stat Med. 2008;27(30):6583–96.
Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res. 2011;2(3):109–12.
Newell DJ. Intention-to-treat analysis: implications for quantitative and qualitative research. Int J Epidemiol. 1992;21(5):837–41.
Montori VM, Guyatt GH. Intention-to-treat principle. CMAJ. 2001;165(10):1339–41.
Abraha I, Montedori A. Modified intention to treat reporting in randomised controlled trials: systematic review. The BMJ. 2010;340:c2697.
Abraha I, Cherubini A, Cozzolino F, De Florio R, Luchetta ML, Rimland JM, et al. Deviation from intention to treat analysis in randomised trials and treatment effect estimates: meta-epidemiological study. BMJ. 2015;350:h2445
Schulz KF, Grimes DA. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet. 2002;359(9308):781–5.
Akl EA, Briel M, You JJ, Sun X, Johnston BC, Busse JW, et al. Potential impact on estimated treatment effects of information lost to follow-up in randomised controlled trials (LOST-IT): systematic review. BMJ. 2012;344:e2809.
Rerkasem K, Rothwell PM. Meta-analysis of small randomized controlled trials in surgery may be unreliable. Br J Surg. 2010;97(4):466–9.
Abdulatif M, Mukhtar A, Obayah G. Pitfalls in reporting sample size calculation in randomized controlled trials published in leading anaesthesia journals: a systematic review. Br J Anaesth. 2015;115(5):699–707.
Yusuf S, Collins R, Peto R. Why do we need some large, simple randomized trials? Stat Med. 1984;3(4):409–22.
•• Walsh M, Srinathan SK, McAuley DF, Mrkobrada M, Levine O, Ribic C, et al. The statistical significance of randomized controlled trial results is frequently fragile: a case for a Fragility Index. J Clin Epidemiol. 2014;67(6):622–8. - This publication discusses the issue of fragility in trials and introduced the Fragility Index. The Fragility Index is an novel metric that is proposed to complement p-value in assessing statistically significant results reported in trials.
Ridgeon EE, Young PJ, Bellomo R, Mucchetti M, Lembo R, Landoni G. The fragility index in multicenter randomized controlled critical care trials. Crit Care Med. 2016;44(7):1278–84.
Evaniew N, Files C, Smith C, Bhandari M, Ghert M, Walsh M, et al. The fragility of statistically significant findings from randomized trials in spine surgery: a systematic survey. Spine J. 2015;15(10):2188–97.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Emmanuelle Duceppe and Emilie Belley-Coté declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical collection on Research Methods and Statistical Analyses.
Rights and permissions
About this article
Cite this article
Duceppe, E., Belley-Coté, E. An Overview of Challenges and Approaches to Minimize Bias in Randomized Controlled Trials in Perioperative Medicine. Curr Anesthesiol Rep 6, 276–282 (2016). https://doi.org/10.1007/s40140-016-0172-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-016-0172-7