Abstract
Purpose of Review
Docetaxel is an important cancer therapeutic drug that targets microtubules. As a taxane, it has high cytotoxic potential and causes cell death by inducing polymerization of tubulin monomers and inhibiting depolymerization or inducing apoptosis by stimulating the phosphorylation B-cell lymphoma 2 (Bcl-2). As a hydrophobic drug, the clinical application of docetaxel is limited. The nanoparticle-based platforms as drug carriers have opened up the vast potential of docetaxel. The purpose of the review is to introduce the developments in nano-drug carriers in meeting the needs to target individual tumour cellular components.
Recent Findings
The progress from individual nanoparticles like liposome or cubosome to hybrid nanoparticles capable of carrying multiple drugs, targeting them to the tumour site, and releasing the active molecules in a sustained and prolonged manner has been addressed by researchers in recent times. Newer developments of active targeting systems such as through antibody conjugation to the nanoparticle are observed in this area.
Summary
From the knowledge that is currently available in public domain, it can be foreseen that extensive in vivo assessment of the formulations that have shown good prospects is required. The in vivo assessment needs to be based on in vitro models coupled with extensive toxicity studies. This is required to take a larger percentage of the methodologies available in the literature to preclinical and clinical trials and thus to the market. Safe, side effect–free, biocompatible and biodegradable nanoparticles as carriers for docetaxel are around the corner.
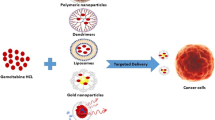
Graphical abstract






Similar content being viewed by others
References
Stout NL, Wagner SS. Antineoplastic therapy side effects and polypharmacy in older adults with cancer. Top Geriatr Rehabil. 2019;35(1):15–30. https://doi.org/10.1097/TGR.0000000000000212.
Sohail MF, Rehman M, Sarwar HS, Naveed S, Salman O, Bukhari NI, et al. Advancements in the oral delivery of docetaxel: challenges, current state-of-the-art and future trends. Int J Nanomedicine. 2018;13:3145–61. https://doi.org/10.2147/ijn.S164518.
Ashtarinezhad A, Panahyab A, Vatanpour H, Shirazi F. FTIR determination of miconazole effects on mice fetus brain tissue. Iranian J Pharm Sci. 2014;10(2):79–84.
Thirumaran R, Prendergast GC, Gilman PB. Chapter 7 - Cytotoxic chemotherapy in clinical treatment of cancer. In: Prendergast GC, Jaffee EM, editors. Cancer Immunotherapy. Burlington: Academic Press; 2007. p. 101–16.
Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature. 2010;463(7280):485–92. https://doi.org/10.1038/nature08908.
Oshiro C, Marsh S, McLeod H, Carrillo MW, Klein T, Altman R. Taxane pathway. Pharmacogenet Genomics. 2009;19(12):979–83. https://doi.org/10.1097/FPC.0b013e3283335277.
Haldar S, Basu A, Croce CM. Bcl2 is the guardian of microtubule integrity. Cancer Res. 1997;57(2):229–33.
Mackler NJ, Pienta KJ. Drug insight: use of docetaxel in prostate and urothelial cancers. Nat Clin Pract Urol. 2005;2(2):92–100.
Eftekhari S, Montazeri H, Tarighi P. Synergistic anti-tumor effects of liraglutide, a glucagon-like peptide-1 receptor agonist, along with Docetaxel on LNCaP prostate cancer cell line. European Journal of Pharmacology. 2020;878:173102.
Jain S, Spandana G, Agrawal AK, Kushwah V, Thanki K. Enhanced antitumor efficacy and reduced toxicity of docetaxel loaded estradiol functionalized stealth polymeric nanoparticles. Mol Pharm. 2015;12(11):3871–84.
Taheri-Ledari R, Zhang W, Radmanesh M, Mirmohammadi SS, Maleki A, Cathcart N, et al. Multi-stimuli nanocomposite therapeutic: docetaxel targeted delivery and synergies in treatment of human breast cancer tumor. Small. 2020;16(41):2002733.
Maloney SM, Hoover CA, Morejon-Lasso LV, Prosperi JR. Mechanisms of taxane resistance. Cancers. 2020;12(11):57. https://doi.org/10.3390/cancers12113323.
Nøhr-Nielsen A, Bagger SO, Brünner N, Stenvang J, Lund TM. Pharmacodynamic modelling reveals synergistic interaction between docetaxel and SCO-101 in a docetaxel-resistant triple negative breast cancer cell line. European Journal of Pharmaceutical Sciences. 2020;148:105315.
Pucci P, Rescigno P, Sumanasuriya S, de Bono J, Crea F. Hypoxia and noncoding RNAs in taxane resistance. Trends Pharmacol Sci. 2018;39(8):695–709. https://doi.org/10.1016/j.tips.2018.05.002.
Guo YF, Zhao S, Qiu HH, Wang T, Zhao YN, Han MH, et al. Shape of nanoparticles as a design parameter to improve docetaxel antitumor efficacy. Bioconjug Chem. 2018;29(4):1302–11. https://doi.org/10.1021/acs.bioconjchem.8b00059.
Panda J, Satapathy BS, Mandal B, Sen R, Mukherjee B, Sarkar R, et al. Anticancer potential of docetaxel-loaded cobalt ferrite nanocarrier: an in vitro study on MCF-7 and MDA-MB-231 cell lines. J Microencapsul. 2021;38(1):36–46. https://doi.org/10.1080/02652048.2020.1842529.
Hu Y, Ran M, Wang B, Lin Y, Cheng Y. Co-delivery of docetaxel and curcumin via nanomicelles for enhancing anti-ovarian cancer treatment. 2020;15:9703-15. https://doi.org/10.2147/ijn.s274083.
Dawoud M, Abourehab MA, Abdou R. Monoolein cubic nanoparticles as novel carriers for docetaxel. J Drug Deliv Sci Technol. Part A. 2020;56:101501. https://doi.org/10.1016/j.jddst.2020.101501.
Dong Z, Shen Y, Zhao S, Wang X, Han M, Zhao N et al. Influence of hydrophobic chains in nanocarriers on antitumor efficacy of docetaxel nanoparticles. 2020;17(4):1205-14. https://doi.org/10.1021/acs.molpharmaceut.9b01228.
Li N, Mai Y, Liu Q, Gou G, Yang J. Docetaxel-loaded D-α-tocopheryl polyethylene glycol-1000 succinate liposomes improve lung cancer chemotherapy and reverse multidrug resistance. Drug Deliv Transl Res. 2021;11(1):131–41. https://doi.org/10.1007/s13346-020-00720-9.
Ding Y, Ding Y, Wang Y, Wang C, Gao M, Xu Y, et al. Soluplus(®)/TPGS mixed micelles for co-delivery of docetaxel and piperine for combination cancer therapy. Pharm Dev Technol. 2020;25(1):107–15. https://doi.org/10.1080/10837450.2019.1679834.
Talkar SS, Kharkar PB, Patravale VB. Docetaxel loaded pomegranate seed oil based nanostructured lipid carriers: a potential alternative to current formulation. AAPS PharmSciTech. 2020;21(8):295. https://doi.org/10.1208/s12249-020-01839-1.
Ghamkhari A, Abbasi F, Abbasi E, Ghorbani M. A novel thermo-responsive system based on β-cyclodextrin-nanocomposite for improving the docetaxel activity. Int J Polym Mater Polym Biomater. 2020:1–11. https://doi.org/10.1080/00914037.2020.1765357.
Gong F, Wang R, Zhu Z, Duan J, Teng X, Cui Z-K. Drug-interactive mPEG-b-PLA-Phe(Boc) micelles enhance the tolerance and anti-tumor efficacy of docetaxel. Drug Delivery. 2020;27(1):238–47. https://doi.org/10.1080/10717544.2020.1718245.
Attia MF, Anton N, Wallyn J, Omran Z, Vandamme TF. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol. 2019;71(8):1185–98. https://doi.org/10.1111/jphp.13098.
He C, Cai P, Li J, Zhang T, Lin L, Abbasi AZ, et al. Blood-brain barrier-penetrating amphiphilic polymer nanoparticles deliver docetaxel for the treatment of brain metastases of triple negative breast cancer. J Control Release. 2017;246:98–109. https://doi.org/10.1016/j.jconrel.2016.12.019.
Gao J, Liu J, Xie F, Lu Y, Yin C, Shen X. Co-delivery of docetaxel and salinomycin to target both breast cancer cells and stem cells by PLGA/TPGS nanoparticles. Int J Nanomedicine. 2019;14:9199–216. https://doi.org/10.2147/IJN.S230376.
Hoang B, Ernsting MJ, Roy A, Murakami M, Undzys E, Li SD. Docetaxel-carboxymethylcellulose nanoparticles target cells via a SPARC and albumin dependent mechanism. Biomaterials. 2015;59:66–76. https://doi.org/10.1016/j.biomaterials.2015.04.032.
Vardhan H, Mittal P, Adena SK, Mishra B. Long-circulating polyhydroxybutyrate-co-hydroxyvalerate nanoparticles for tumor targeted docetaxel delivery: formulation, optimization and in vitro characterization. European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences. 2017;99:85-94. https://doi.org/10.1016/j.ejps.2016.12.007.
Marcinkowska M, Stanczyk M, Janaszewska A, Gajek A, Ksiezak M, Dzialak P, et al. Molecular mechanisms of antitumor activity of PAMAM dendrimer conjugates with anticancer drugs and a monoclonal antibody. Polymers. 2019;11(9):16. https://doi.org/10.3390/polym11091422.
Ashrafizadeh M, Ahmadi Z, Mohamadi N, Zarrabi A, Abasi S, Dehghannoudeh G, et al. Chitosan-based advanced materials for docetaxel and paclitaxel delivery: recent advances and future directions in cancer theranostics. Int J Biol Macromol. 2020;145:282–300. https://doi.org/10.1016/j.ijbiomac.2019.12.145.
Mirzaie ZH, Irani S, Mirfakhraie R, Atyabi SM, Dinarvand M, Dinarvand R, et al. Docetaxel-chitosan nanoparticles for breast cancer treatment: cell viability and gene expression study. Chem Biol Drug Des. 2016;88(6):850–8. https://doi.org/10.1111/cbdd.12814.
Wang H, Xu Y, Zhou X. Docetaxel-loaded chitosan microspheres as a lung targeted drug delivery system: in vitro and in vivo evaluation. Int J Mol Sci. 2014;15(3):3519–32. https://doi.org/10.3390/ijms15033519.
Lee E, Kim H, Lee I-H, Jon S. In vivo antitumor effects of chitosan-conjugated docetaxel after oral administration. J Control Release. 2009;140(2):79–85. https://doi.org/10.1016/j.jconrel.2009.08.014.
Hwang H-Y, Kim I-S, Kwon IC, Kim Y-H. Tumor targetability and antitumor effect of docetaxel-loaded hydrophobically modified glycol chitosan nanoparticles. J Control Release. 2008;128(1):23–31. https://doi.org/10.1016/j.jconrel.2008.02.003.
Mu C-F, Cui F, Yin Y-M, Cho H-J, Kim D-D. Docetaxel-loaded chitosan-cholesterol conjugate-based self-assembled nanoparticles for overcoming multidrug resistance in cancer cells. Pharmaceutics. 2020;12(9):783.
Youm I, Agrahari V, Murowchick JB, Youan B-BC. Uptake and cytotoxicity of docetaxel-loaded hyaluronic acid-grafted oily core nanocapsules in MDA-MB 231 cancer cells. Pharm Res. 2014;31(9):2439–52.
Zhu Z, Li Y, Yang X, Pan W, Pan H. The reversion of anti-cancer drug antagonism of tamoxifen and docetaxel by the hyaluronic acid-decorated polymeric nanoparticles. Pharmacol Res. 2017;126:84–96. https://doi.org/10.1016/j.phrs.2017.07.011.
He L, Shang Z, Liu H, Yuan Z-x. Alginate-based platforms for cancer-targeted drug delivery. Biomed Res Int. 2020;2020:1487259–17. https://doi.org/10.1155/2020/1487259.
Zhang P, Chen L, Zhang Z, Lin L, Li Y. Pharmacokinetics in rats and efficacy in murine ovarian cancer model for solid lipid nanoparticles loading docetaxel. J Nanosci Nanotechnol. 2010;10(11):7541–4. https://doi.org/10.1166/jnn.2010.2819.
da Rocha MCO, da Silva PB, Radicchi MA, Andrade BYG, de Oliveira JV, Venus T, et al. Docetaxel-loaded solid lipid nanoparticles prevent tumor growth and lung metastasis of 4 T1 murine mammary carcinoma cells. J Nanobiotechnology. 2020;18(1):43. https://doi.org/10.1186/s12951-020-00604-7.
Kim KS, Youn YS, Bae YH. Immune-triggered cancer treatment by intestinal lymphatic delivery of docetaxel-loaded nanoparticle. Journal of controlled release : official journal of the Controlled Release Society. 2019;311-312:85-95. doi:10.1016/j.jconrel.2019.08.027.
Kothari IR, Mazumdar S, Sharma S, Italiya K, Mittal A, Chitkara D. Docetaxel and alpha-lipoic acid co-loaded nanoparticles for cancer therapy. Ther Deliv. 2019;10(4):227–40. https://doi.org/10.4155/tde-2018-0074.
Vakili-Ghartavol R, Rezayat SM, Faridi-Majidi R, Sadri K, Jaafari MR. Optimization of docetaxel loading conditions in Liposomes: proposing potential products for metastatic breast carcinoma chemotherapy. Sci Rep. 2020;10(1):5569. https://doi.org/10.1038/s41598-020-62501-1.
Hua H, Zhang N, Liu D, Song L, Liu T, Li S, et al. Multifunctional gold nanorods and docetaxel-encapsulated liposomes for combined thermo- and chemotherapy. Int J Nanomedicine. 2017;12:7869–84. https://doi.org/10.2147/ijn.s143977.
Chowdhury N, Vhora I, Patel K, Doddapaneni R, Mondal A, Singh M. Liposomes co-loaded with 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 3 (PFKFB3) shRNA plasmid and docetaxel for the treatment of non-small cell lung cancer. Pharm Res. 2017;34(11):2371–84. https://doi.org/10.1007/s11095-017-2244-x.
Pereira S, Egbu R, Jannati G, Al-Jamal WT. Docetaxel-loaded liposomes: the effect of lipid composition and purification on drug encapsulation and in vitro toxicity. Int J Pharm. 2016;514(1):150–9. https://doi.org/10.1016/j.ijpharm.2016.06.057.
Kushwah V, Jain DK, Agrawal AK, Jain S. Improved antitumor efficacy and reduced toxicity of docetaxel using anacardic acid functionalized stealth liposomes. Colloids Surf B: Biointerfaces. 2018;172:213–23. https://doi.org/10.1016/j.colsurfb.2018.08.047.
Ranjan A, Benjamin CJ, Negussie AH, Chokshi S, Chung PH, Volkin D, et al. Biodistribution and efficacy of low temperature-sensitive liposome encapsulated docetaxel combined with mild hyperthermia in a mouse model of prostate cancer. Pharm Res. 2016;33(10):2459–69. https://doi.org/10.1007/s11095-016-1971-8.
Belz J, Castilla-Ojo N, Sridhar S, Kumar R. Radiosensitizing silica nanoparticles encapsulating docetaxel for treatment of prostate cancer. In: Zeineldin R, editor. Cancer Nanotechnology: Methods and Protocols. New York, NY: Springer New York; 2017. p. 403–9.
Li L, Tang F, Liu H, Liu T, Hao N, Chen D, et al. In vivo delivery of silica nanorattle encapsulated docetaxel for liver cancer therapy with low toxicity and high efficacy. ACS Nano. 2010;4(11):6874–82. https://doi.org/10.1021/nn100918a.
Ao M, Xiao X, Ao Y. Low density lipoprotein modified silica nanoparticles loaded with docetaxel and thalidomide for effective chemotherapy of liver cancer. Braz J Med Biol Res. 2018;51(3): e6650. https://doi.org/10.1590/1414-431X20176650.
Hao Y, Zheng C, Song Q, Chen H, Nan W, Wang L, et al. Pressure-driven accumulation of Mn-doped mesoporous silica nanoparticles containing 5-aza-2-deoxycytidine and docetaxel at tumours with a dry cupping device. J Drug Target. 2021:1–10. https://doi.org/10.1080/1061186X.2021.1892117.
Thambiraj S, Shruthi S, Vijayalakshmi R, Ravi SD. Evaluation of cytotoxic activity of docetaxel loaded gold nanoparticles for lung cancer drug delivery. Cancer Treat Res Commun. 2019;21:100157. https://doi.org/10.1016/j.ctarc.2019.100157.
Wan J, Ma X, Xu D, Yang B, Yang S, Han S. Docetaxel-decorated anticancer drug and gold nanoparticles encapsulated apatite carrier for the treatment of liver cancer. J Photochem Photobiol B. 2018;185:73–9. https://doi.org/10.1016/j.jphotobiol.2018.05.021.
Raposo LR, Roma-Rodrigues C, Jesus J, Martins L, Pombeiro AJ, Baptista PV, et al. Targeting canine mammary tumours via gold nanoparticles functionalized with promising Co(II) and Zn(II) compounds. Vet Comp Oncol. 2017;15(4):1537–42. https://doi.org/10.1111/vco.12298.
François A, Laroche A, Pinaud N, Salmon L, Ruiz J, Robert J, et al. Encapsulation of docetaxel into PEGylated gold nanoparticles for vectorization to cancer cells. ChemMedChem. 2011;6(11):2003–8. https://doi.org/10.1002/cmdc.201100311.
Wang H, Zhao R, Li Y, Liu H, Li F, Zhao Y, et al. Aspect ratios of gold nanoshell capsules mediated melanoma ablation by synergistic photothermal therapy and chemotherapy. Nanomed Nanotechnol Biol Med. 2016;12(2):439–48. https://doi.org/10.1016/j.nano.2015.11.013.
Huang X, Yi C, Fan Y, Zhang Y, Zhao L, Liang Z, et al. Magnetic Fe3 O 4 nanoparticles grafted with single-chain antibody (scFv) and docetaxel loaded β-cyclodextrin potential for ovarian cancer dual-targeting therapy. Mater Sci Eng C Mater Biol Appl. 2014;42:325–32. https://doi.org/10.1016/j.msec.2014.05.041.
Xie W, Gao Q, Guo Z, Wang D, Gao F, Wang X. Injectable and self-healing thermosensitive magnetic hydrogel for asynchronous control release of doxorubicin and docetaxel to treat triple-negative breast cancer. 2017;9(39):33660-73. https://doi.org/10.1021/acsami.7b10699.
Taheri-Ledari R, Zhang W, Radmanesh M, Mirmohammadi SS, Maleki A. Multi-stimuli nanocomposite therapeutic: docetaxel targeted delivery and synergies in treatment of human breast cancer tumor. 2020;16(41):e2002733. https://doi.org/10.1002/smll.202002733.
Sato A, Itcho N, Ishiguro H, Okamoto D, Kobayashi N, Kawai K, et al. Magnetic nanoparticles of Fe3O4 enhance docetaxel-induced prostate cancer cell death. Int J Nanomedicine. 2013;8(1):3151–60. https://doi.org/10.2147/ijn.s40766.
Du B, Han S, Li H, Zhao F, Su X, Cao X, et al. Multi-functional liposomes showing radiofrequency-triggered release and magnetic resonance imaging for tumor multi-mechanism therapy. Nanoscale. 2015;7(12):5411–26. https://doi.org/10.1039/c4nr04257c.
Yang Q, Chen H, Bai Y, Cao Y, Hu W, Zhang L. Facile synthesis of lipid-perfluorocarbon nanoemulsion coated with silica shell as an ultrasound imaging agent. 2018;7(4). https://doi.org/10.1002/adhm.201700816.
Wu R, Zhang Z, Wang B, Chen G, Zhang Y, Deng H, et al. Combination chemotherapy of lung cancer - co-delivery of docetaxel prodrug and cisplatin using aptamer-decorated lipid-polymer hybrid nanoparticles. Drug Des Devel Ther. 2020;14:2249–61. https://doi.org/10.2147/dddt.s246574.
Lee HS, Kang NW, Kim H, Kim DH, Chae JW, Lee W, et al. Chondroitin sulfate-hybridized zein nanoparticles for tumor-targeted delivery of docetaxel. Carbohydr Polym. 2021;253:117187. https://doi.org/10.1016/j.carbpol.2020.117187.
Yang Q, Liu DZ, Liu M, Ji QF, Mei QB, Cheng Y, et al. Bone-targeted calcium phosphate-polymer hybrid nanoparticle co-deliver zoledronate and docetaxel to treat bone metastasis of prostate cancer. J Pharm Sci. 2021;110(2):876–87. https://doi.org/10.1016/j.xphs.2020.11.005.
Han J, Zhen J, Du Nguyen V, Go G, Choi Y, Ko SY, et al. Hybrid-actuating macrophage-based microrobots for active cancer therapy. Sci Rep. 2016;6:28717. https://doi.org/10.1038/srep28717.
Gao J, Liu J. Co-delivery of docetaxel and salinomycin to target both breast cancer cells and stem cells by PLGA/TPGS nanoparticles. Int J Nanomedicine. 2019;14:9199-216. doi:https://doi.org/10.2147/ijn.s230376.
Chen L, Zhou L, Wang C, Han Y, Lu Y, Liu J, et al. Tumor-targeted drug and CpG delivery system for phototherapy and docetaxel-enhanced immunotherapy with polarization toward M1-type macrophages on triple negative breast cancers. 2019;31(52):e1904997. 10.1002/adma.201904997.
Zhao D, Jiang K, Wang YQ, Cheng J, Mo FL, Luo T, et al. Out-of-the-box nanocapsules packed with on-demand hydrophobic anticancer drugs for lung targeting, esterase triggering, and synergy therapy. Adv Healthc Mater. 9. 10.1002/adhm.202001803.
Sung SY, Su YL, Cheng W, Hu PF, Chiang CS, Chen WT, et al. Graphene quantum dots-mediated theranostic penetrative delivery of drug and photolytics in deep tumors by targeted biomimetic nanosponges. Nano Lett. 2019;19(1):69–81. https://doi.org/10.1021/acs.nanolett.8b03249.
Sumer Bolu B, Golba B. Trastuzumab targeted micellar delivery of docetaxel using dendron-polymer conjugates 2020;8(9):2600-10. https://doi.org/10.1039/c9bm01764j.
Ashrafizadeh M, Zarrabi A, Hashemi F, Zabolian A, Saleki H, Bagherian M, et al. Polychemotherapy with curcumin and doxorubicin via biological nanoplatforms: enhancing antitumor activity. Pharmaceutics. 2020;12(11):33. https://doi.org/10.3390/pharmaceutics12111084.
Hu YZ, Ran MN, Wang BL, Lin YZ, Cheng YZ, Zheng SP. Co-delivery of docetaxel and curcumin via nanomicelles for enhancing anti-ovarian cancer treatment. Int J Nanomed. 2020;15:9703–15. https://doi.org/10.2147/ijn.S274083.
Deng L, Zhu X, Yu Z, Li Y, Qin L, Liu Z, et al. Novel T7-Modified pH-responsive targeted nanosystem for co-delivery of docetaxel and curcumin in the treatment of esophageal cancer. Int J Nanomedicine. 2020;15:7745–62. https://doi.org/10.2147/IJN.S257312.
Rawal S, Bora V, Patel B, Patel M. Surface-engineered nanostructured lipid carrier systems for synergistic combination oncotherapy of non-small cell lung cancer. Drug Deliv Transl Res. 22. 10.1007/s13346-020-00866-6.
Jackson JK, Letchford K. The effective solubilization of hydrophobic drugs using epigallocatechin gallate or tannic acid-based formulations. J Pharm Sci. 2016;105(10):3143–52. https://doi.org/10.1016/j.xphs.2016.06.027.
Bharali DJ, Sudha T, Cui H, Mian BM, Mousa SA. Anti-CD24 nano-targeted delivery of docetaxel for the treatment of prostate cancer. Nanomed Nanotechnol Biol Med. 2017;13(1):263–73. https://doi.org/10.1016/j.nano.2016.08.017.
Nasrollahi F, Varshosaz J, Khodadadi AA, Lim S, Jahanian-Najafabadi A. Targeted delivery of docetaxel by use of transferrin/poly(allylamine hydrochloride)-functionalized graphene oxide nanocarrier. ACS applied materials & interfaces. 2016;8(21):13282–93. https://doi.org/10.1021/acsami.6b02790.
Razi Soofiyani S, Mohammad Hoseini A, Mohammadi A, Khaze Shahgoli V, Baradaran B, Hejazi MS. siRNA-mediated silencing of CIP2A enhances docetaxel activity against PC-3 prostate cancer cells. Adv Pharm Bull. 2017;7(4):637–43. https://doi.org/10.15171/apb.2017.076.
Bahreyni A, Luo HL. Advances in targeting cancer-associated genes by designed siRNA in prostate cancer. Cancers. 2020;12(12):3619. https://doi.org/10.3390/cancers12123619.
Imran M, Saleem S, Chaudhuri A, Ali J, Baboota S. Docetaxel: an update on its molecular mechanisms, therapeutic trajectory and nanotechnology in the treatment of breast, lung and prostate cancer. J Drug Deliv Sci Technol. 2020;60:101959. https://doi.org/10.1016/j.jddst.2020.101959.
Vermunt MAC, Bergman AM, van der Putten E, Beijnen JH. The intravenous to oral switch of taxanes: strategies and current clinical developments. Future Oncol. 2021;7(11):1379–99. https://doi.org/10.2217/fon-2020-0876.
McEntee M, Silverman JA, Rassnick K, Zgola M, Chan AO, Tau PT, et al. Enhanced bioavailability of oral docetaxel by co-administration of cyclosporin A in dogs and rats. Vet Comp Oncol. 2003;1(2):105–12. https://doi.org/10.1046/j.1476-5829.2003.00015.x.
Adityan S, Tran M, Bhavsar C, Wu SY. Nano-therapeutics for modulating the tumour microenvironment: design, development, and clinical translation. J Control Release. 2020;327:512–32. https://doi.org/10.1016/j.jconrel.2020.08.016.
Anselmo AC, Mitragotri S. Nanoparticles in the clinic: an update. Bioeng Transl Med. 2019;4(3):16. https://doi.org/10.1002/btm2.10143.
Di Maio M, Perrone F, Conte P. Real-world evidence in oncology: opportunities and limitations. Oncologist. 2020;25(5):E746–E52. https://doi.org/10.1634/theoncologist.2019-0647.
Acknowledgements
SM and SN thank Chettinad Academy of Research and Education for CARE JRF. The authors thank the infrastructure support provided by Chettinad Academy of Research and Education.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors attest that we have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Nanoparticle-based Drug Delivery
Rights and permissions
About this article
Cite this article
Manivannan, S., Nagaraj, S. & Narayan, S. A Reflection on the Mechanism of the Role of Nanoparticles in Increasing the Efficacy of Anti-tumour Properties of Docetaxel. Curr Pathobiol Rep 9, 79–91 (2021). https://doi.org/10.1007/s40139-021-00223-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40139-021-00223-3